You have no items in your shopping cart
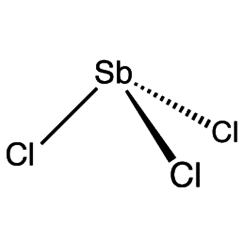
Antimony trichloride
Antimony trichloride is the chemical compound with the formula SbCl3. The soft colorless solid with a pungent odor was known to the alchemists as butter of antimony.
SbCl3 is a reagent for detecting vitamin A and related carotenoids in the Carr-Price test. The antimony trichloride reacts with the carotenoid to form a blue complex that can be measured by colorimetry.
Antimony trichloride has also been used as an adulterant to enhance the louche effect in absinthe. It has been used in the past to dissolve and remove horn stubs from calves without having to cut them off.
It is also used as a catalyst for polymerization, hydrocracking, and chlorination reactions; as a mordant; and in the production of other antimony salts. Its solution is used as an analytical reagent for chloral, aromatics, and vitamin A. It has a very potential use as a Lewis acid catalyst in synthetic organic transformation.
A solution of antimony trichloride in liquid hydrogen sulfide is a good conductor, though the applications of such are limited by the very low temperature or high pressure required for hydrogen sulfide to be liquid