You have no items in your shopping cart
Malic acid
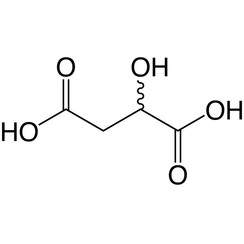
Malic acid is an organic compound with the molecular formula C4H6O5. It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms (L- and D-enantiomers), though only the L-isomer exists naturally. The salts and esters of malic acid are known as malates. The malate anion is an intermediate in the citric acid cycle.
Malic acid was first isolated from apple juice by Carl Wilhelm Scheele in 1785. Antoine Lavoisier in 1787 proposed the name acide malique, which is derived from the Latin word for apple, mālum—as is its genus name Malus. In German it is named Äpfelsäure (or Apfelsäure) after plural or singular of the fruit apple, but the salt(s) Malat(e). Malic acid is the main acid in many fruits, including apricots, blackberries, blueberries, cherries, grapes, mirabelles, peaches, pears, plums, and quince and is present in lower concentrations in other fruits, such as citrus. It contributes to the sourness of unripe apples. Sour apples contain high proportions of the acid. It is present in grapes and in most wines with concentrations sometimes as high as 5 g/l. It confers a tart taste to wine; the amount decreases with increasing fruit ripeness. The taste of malic acid is very clear and pure in rhubarb, a plant for which it is the primary flavor. It is also a component of some artificial vinegar flavors, such as "salt and vinegar" flavored potato chips.
In citrus, fruits produced in organic farming contain higher levels of malic acid than fruits produced in conventional agriculture.
The process of malolactic fermentation converts malic acid to much milder lactic acid. Malic acid occurs naturally in all fruits and many vegetables, and is generated in fruit metabolism.
Malic acid, when added to food products, is denoted by E number E296. Malic acid is the source of extreme tartness in United States-produced confectionery, the so-called extreme candy. It is also used with or in place of the less sour citric acid in sour sweets. These sweets are sometimes labeled with a warning stating that excessive consumption can cause irritation of the mouth. It is approved for use as a food additive in the EU, US and Australia and New Zealand (where it is listed by its INS number 296).
Malic acid provides 10 kJ (2.39 kilocalories) of energy per gram during digestion.