You have no items in your shopping cart
Ethylene carbonate
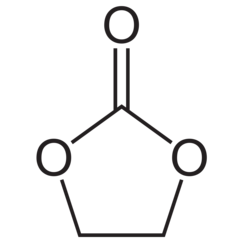
Ethylene carbonate (sometimes abbreviated EC) is the organic compound with the formula (CH2O)2CO. It is classified as the carbonate ester of ethylene glycol and carbonic acid. At room temperature (25 °C) ethylene carbonate is a transparent crystalline solid, practically odorless and colorless, and somewhat soluble in water. In the liquid state (m.p. 34-37 °C) it is a colorless odorless liquid.
Ethylene carbonate is used as a polar solvent with a molecular dipole moment of 4.9 D, only 0.1 D lower than that of propylene carbonate.
It can be used as a high permittivity component of electrolytes in lithium batteries and lithium-ion batteries. Other components like diethyl carbonate, ethyl methyl carbonate, dimethyl carbonate and methyl acetate can be added to those electrolytes in order to decrease the viscosity and melting point.
Ethylene carbonate is also used as plasticizer, and as a precursor to vinylene carbonate, which is used in polymers and in organic synthesis.