You have no items in your shopping cart
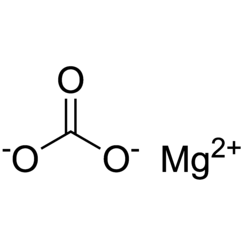
Magnesium carbonate
The primary use of magnesium carbonate is the production of magnesium oxide by calcining. Magnesite and dolomite minerals are used to produce refractory bricks.MgCO3 is also used in flooring, fireproofing, fire extinguishing compositions, cosmetics, dusting powder, and toothpaste. Other applications are as filler material, smoke suppressant in plastics, a reinforcing agent in neoprene rubber, a drying agent, a laxative to loosen the bowels, and colour retention in foods. In addition, high purity magnesium carbonate is used as antacid and as an additive in table salt to keep it free flowing. Magnesium carbonate can do this because it doesn't dissolve in water, only acid, where it will effervesce (bubble).
Because of its low solubility in water and hygroscopic properties, MgCO3 was first added to salt in 1911 to make it flow more freely. The Morton Salt company adopted the slogan "When it rains it pours" with reference to the fact that its MgCO3-containing salt would not stick together in humid weather.Magnesium carbonate, most often referred to as "chalk", is also used as a drying agent on athletes' palms in rock climbing, gymnastics, and weight lifting.
As a food additive magnesium carbonate is known as E504, for which the only known side effect is that it may work as a laxative in high concentrations.
Magnesium carbonate is also used in taxidermy for whitening skulls. It can be mixed with hydrogen peroxide to create a paste, which is then spread on the skull to give it a white finish.
In addition, magnesium carbonate is used as a matte white coating for projection screens