You have no items in your shopping cart
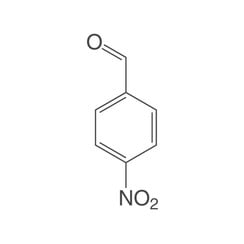
4-Nitrobenzaldehyde
In chemistry, nitrobenzaldehydes are a group of substances derived from both benzaldehyde and nitrobenzene. The structure consists of a benzene ring with attached aldehyde (–CHO) and nitro groups (–NO2). Their different arrangement (ortho, meta or para) results in three constitutional isomers with the empirical formula C7H5NO3.
The nitrobenzaldehydes are pale yellow crystalline solids, some of which have a bitter almond odor. The 4-nitrobenzaldehyde, which has the highest symmetry, has the highest melting point.
In a base medium, the nitrobenzaldehydes are disproportionate in a Cannizzaro reaction to form nitrobenzoic acids and nitrobenzyl alcohols.
Application
2-Nitrobenzaldehyde is used as a reagent on isopropanol and acetone.
2-Nitrobenzaldehyde reacts with acetone to form indigo.