You have no items in your shopping cart
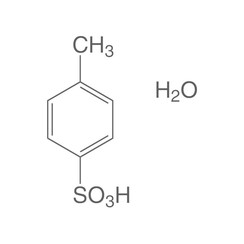
p-Toluenesulphonic acid
p-Toluenesulfonic acid (PTSA or pTsOH) or tosylic acid (TsOH) is an organic compound with the formula CH3C6H4SO3H. It is a white solid that is soluble in water, alcohols, and other polar organic solvents. The CH3C6H4SO2– group is known as the tosyl group and is often abbreviated as Ts or Tos. Most often, TsOH refers to the monohydrate, TsOH.H2O.
As with other sulfonic acids, TsOH is a strong organic acid. It is about one million times stronger than benzoic acid. It is one of the few strong acids that is solid and, hence, conveniently weighed.
TsOH is prepared on an industrial scale by the sulfonation of toluene. It hydrates readily. Common impurities include benzenesulfonic acid and sulfuric acid. Monohydrate pTSA contains crystalline water as well as water as Impurity. To estimate the total moisture present as impurity, Karl Fischer method is used. Impurities can be removed by recrystallization from its concentrated aqueous solution followed by azeotropic drying with toluene.
TsOH finds use in organic synthesis as an "organic-soluble" acid catalyst. Examples of uses include:
- Acetalization of an aldehyde.
- Esterification of carboxylic acids.
- Transesterification of an ester.