You have no items in your shopping cart
Trypsin
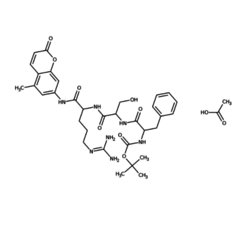
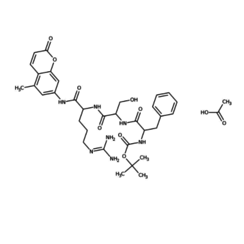
Trypsin is a pancreatic juice peptidase involved in the digestion of proteins. It is a serine protease that hydrolyzes the peptide bonds located on the C side of a lysine or arginine residue, which are basic amino acids.
Trypsin is found in most animals. This enzyme is widely used for proteomics research approaches, especially for the characterization and sequencing of proteins. Thus, it is mainly used in mass spectrometry to digest proteins before analysis.
Trypsin is used in cell culture to release cells that adhere to culture flasks or Petri dishes. In effect, trypsin cleaves the adhesion membrane proteins and the cells are then found in suspension. This "trypsinization" is used daily to maintain cell cultures (by switching to a larger culture flask or by multiplying the flasks), to count cells by flow cytometry, or to perform other analyzes. However, this treatment affects the cells a little (viability, membrane markers), so the action of trypsin must be limited: it is eliminated by washing the cells, it is also inhibited by the addition of fetal calf serum (proteins in great excess of membrane proteins ), or a specific inhibitor 2. Alternatively, other enzymes are used3.
Trypsin is also used in immunohematology for the detection of irregular antibodies or even during the establishment of a karyotype: for example, by hydrolyzing histones, and in combination with staining by Giemsa, it allows the appearance of G bands on the DNA and thus the precise identification of chromosomes. This way a chromosomal abnormality can be detected.
-Synthesis
It is synthesized by the pancreas as trypsinogen (inactive proenzyme) and then stored in the enzymatic vesicles of acinar cells from which it is secreted during digestion. Activation of trypsinogen to trypsin results from hydrolysis of the propeptide under the influence of enterokinase or by a self-activating effect of trypsin. Cholecystokinin pancreozymin activates the secretion of enzymes (i.e. trypsin) in pancreatic juice.