You have no items in your shopping cart
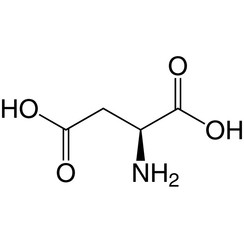
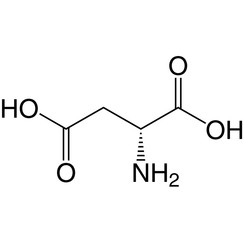
Aspartic acid
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. Like all other amino acids, it contains an amino group and a carboxylic acid. Its α-amino group is in the protonated –NH+
3 form under physiological conditions, while its α-carboxylic acid group is deprotonated −COO− under physiological conditions. Aspartic acid has an acidic side chain (CH2COOH) which reacts with other amino acids, enzymes and proteins in the body.[5] Under physiological conditions (pH 7.4) in proteins the side chain usually occurs as the negatively charged aspartate form, −COO−. It is a non-essential amino acid in humans, meaning the body can synthesize it as needed. It is encoded by the codons GAU and GAC.
D-Aspartate is one of two D-amino acids commonly found in mammals.
In proteins aspartate sidechains are often hydrogen bonded to form asx turns or asx motifs, which frequently occur at the N-termini of alpha helices.
The L-isomer of Asp is one of the 22 proteinogenic amino acids, i.e., the building blocks of proteins. Aspartic acid, like glutamic acid, is classified as an acidic amino acid, with a pKa of 3.9, however in a peptide this is highly dependent on the local environment, and could be as high as 14. Asp is pervasive in biosynthesis. Because aspartate can be synthesized by the body it is classified as a non-essential amino acid.