You have no items in your shopping cart
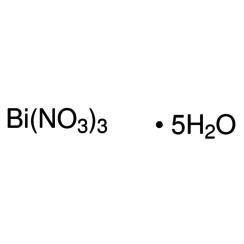
Bismuth(III) nitrate
Bismuth (III) nitrate is a salt composed of bismuth in its cationic +3 oxidation state and nitrate anions. The most common solid form is the pentahydrate. It is used in the synthesis of other bismuth compounds. It is available commercially. It is the only nitrate salt formed by a Group 15 element, which is an indication of the metallic nature of bismuth.
Bismuth nitrate can be prepared by the reaction of bismuth metal and concentrated nitric acid.
Bi + 4HNO3 → Bi (NO3) 3 + 2H2O + NO
It dissolves in nitric acid, but is easily hydrolyzed to form a series of oxynitrates when the pH rises above 0.
It is also soluble in acetone, acetic acid and glycerol, but practically insoluble in ethanol and ethyl acetate.
Some applications in organic synthesis have been reported, for example the nitration of aromatics and selective oxidation of sulfides to sulfoxides. It is also used to form Dragendorff reagent, which is used as a TLC stain.
Bismuth nitrate forms insoluble complexes with pyrogallol and cupferron and these have been the basis of gravimetric methods for determining the bismuth content.
When heated, bismuth nitrate can decompose to form nitrogen dioxide, NO2.