You have no items in your shopping cart
Cysteine
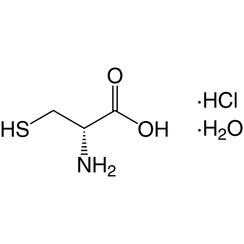
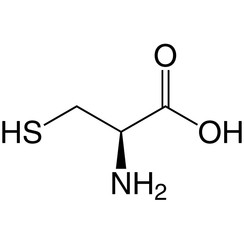
Cysteine is a semiessential proteinogenic amino acid with the formula HO2CCH(NH2)CH2SH. The thiol side chain in cysteine often participates in enzymatic reactions, as a nucleophile. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. When used as a food additive, it has the E number E920. It is encoded by the codons UGU and UGC.
Cysteine has the same structure as serine, but with one of its oxygen atoms replaced by sulfur; replacing it with selenium gives selenocysteine. Like other natural proteinogenic amino acids, cysteine has l chirality in the older d/l notation based on homology to d- and l-glyceraldehyde. In the newer R/S system of designating chirality, based on the atomic numbers of atoms near the asymmetric carbon, cysteine (and selenocysteine) have R chirality, because of the presence of sulfur (or selenium) as a second neighbour to the asymmetric carbon. The remaining chiral amino acids, having lighter atoms in that position, have S chirality.