You have no items in your shopping cart
Formic acid
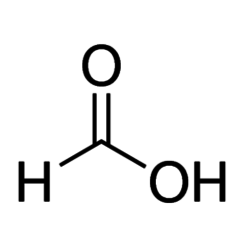
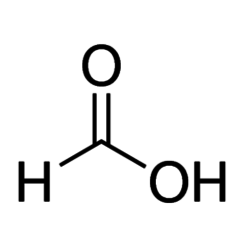
Formic acid, systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH. The chemical composition is HCOOH. It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. The word "formic" comes from the Latin word for ant, formica, referring to its early isolation by the distillation of ant bodies. Esters, salts, and the anion derived from formic acid are called formates. Industrially, formic acid is produced from methanol.
A major use of formic acid is as a preservative and antibacterial agent in livestock feed. In Europe, it is applied on silage, including fresh hay, to promote the fermentation of lactic acid and to suppress the formation of butyric acid; it also allows fermentation to occur quickly, and at a lower temperature, reducing the loss of nutritional value. Formic acid arrests certain decay processes and causes the feed to retain its nutritive value longer, and so it is widely used to preserve winter feed for cattle. In the poultry industry, it is sometimes added to feed to kill E. coli bacteria.[26][27] Use as preservative for silage and (other) animal feed constituted 30% of the global consumption in 2009.
Formic acid is also significantly used in the production of leather, including tanning (23% of the global consumption in 2009), and in dyeing and finishing textiles (9% of the global consumption in 2009) because of its acidic nature. Use as a coagulant in the production of rubber consumed 6% of the global production in 2009.
Formic acid is also used in place of mineral acids for various cleaning products, such as limescale remover and toilet bowl cleaner. Some formate esters are artificial flavorings and perfumes.
Formic acid application has been reported to be an effective treatment for warts.
Formic acid can be used as a fuel cell (it can be used directly in formic acid fuel cells and indirectly in hydrogen fuel cells).
It is possible to use formic acid as an intermediary to produce isobutanol from CO2 using microbes
Formic acid is often used as a component of mobile phase in reversed-phase high-performance liquid chromatography (RP-HPLC) analysis and separation techniques for the separation of hydrophobic macromolecules, such as peptides, proteins and more complex structures including intact viruses. Especially when paired with mass spectrometry detection, formic acid offers several advantages over the more traditionally used phosphoric acid.