You have no items in your shopping cart
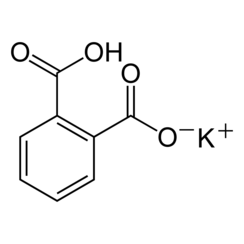
Potassium hydrogen phthalate
Potassium hydrogen phthalate, often called simply KHP, is an acidic salt compound. It forms white powder, colorless crystals, a colorless solution, and an ionic solid that is the monopotassium salt of phthalic acid. KHP is slightly acidic, and it is often used as a primary standard for acid-base titrations because it is solid and air-stable, making it easy to weigh accurately. It is not hygroscopic.[3][4][5] It is also used as a primary standard for calibrating pH meters because, besides the properties just mentioned, its pH in solution is very stable. It also serves as a thermal standard in thermogravimetric analysis[6].
KHP dissociates completely in water, giving the potassium cation (K+) and hydrogen phthalate anion (HP− or Hphthalate−).
- KHP + H2O ⇌ K+ + HP−
And then as a weak acid hydrogen phthalate reacts reversibly with water to give hydronium (H3O+) and phthalate ions.
- HP− + H2O ⇌ P2− + H3O+
KHP can be used as a buffering agent in combination with hydrochloric acid (HCl) or sodium hydroxide (NaOH) depending on which side of pH 4.0 the buffer is to be.
KHP is also a useful standard for total organic carbon (TOC) testing. Most TOC analyzers are based on the oxidation of organics to carbon dioxide and water, with subsequent quantitation of the carbon dioxide. Many TOC analysts suggest testing their instruments with two standards: one typically easy for the instrument to oxidize (KHP), and one more difficult to oxidize. For the latter, benzoquinone is suggested.