You have no items in your shopping cart
Sodium peroxide
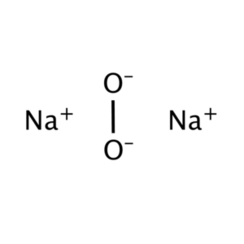
Sodium peroxide is the inorganic compound with the formula Na2O2. This yellowish solid is the product of sodium ignited in excess oxygen. It's a strong foundation. This metal peroxide exists in several hydrates and peroxyhydrates, including Na2O2 2H2O2 4H2O, Na2O2 2H2O, Na2O2 2H2O2 and Na2O2 8H2O. The easy-to-prepare octahydrate is white, unlike the anhydrous material.
Sodium peroxide hydrolyzes to give sodium hydroxide and hydrogen peroxide according to the reaction
Na2O2 + 2 H2O → 2 NaOH + H2O2
Sodium peroxide was used to bleach wood pulp for paper and textile production. Currently it is mainly used for specialized laboratory operations, for example for the extraction of minerals from various ores. Sodium peroxide may bear the commercial names Solozone and Flocool. Sodium peroxide is used as an oxidizing agent in chemical preparations. It is also used as a source of oxygen by reacting it with carbon dioxide to produce oxygen and sodium carbonate:
2 Na2O2 + 2 CO2 → 2 Na2CO3 + O2
Thus, it is particularly useful in scuba gear, submarines, etc. Lithium peroxide has similar uses.