You have no items in your shopping cart
Hydroquinone ≥99.8%, extra pure
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Hydrohinone?
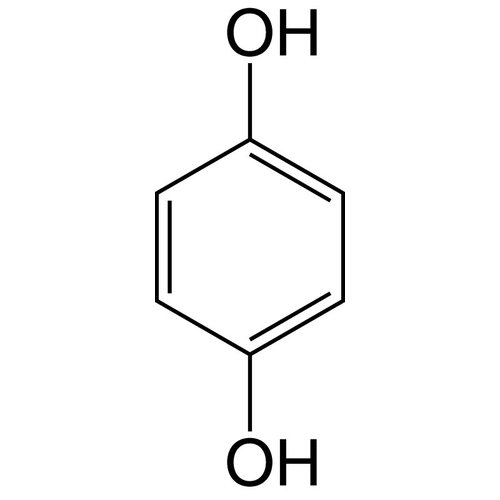
Hydroquinone, also called benzene-1,4-diol, is an aromatic organic compound from the family of polyphenols and diphenols, with the following formula C6H4(OH)2 that appears as a solid under normal temperature and pressure conditions.
The structure has two hydroxyl groups bonded to a benzene ring in the para position.
What is hydroquinone used for?
In the early days of human medicine, it was applied to the skin to reduce the brown pigment load, thereby lightening the skin. Due to abuse this is no longer allowed and only available in the form of a magistral preparation prescribed by the dermatologist. Since February 2001 there are no more cosmetic specialties containing hydroquinone in Europe.
Hydroquinone in aqueous solution has many uses, mainly due to its action as a reducing agent. It is one of the key components in photographic development, reducing exposed, invisible silver salts to metallic silver in the presence of metol (or 4-(methylamino)phenol).
The typical polyether ether ketone (PEEK) synthesis reaction involves 4,4'-difluorobenzophenone and the disodium salt of hydroquinone, which is generated in situ by deprotonation with sodium carbonate. The reaction takes place at about 300°C in polar aprotic solvents such as diphenyl sulfone
In polymer synthesis it is also used as an inhibitor to prevent premature polymerization of the monomer, for example by oxygen in the air (it is a biradical that can initiate polymerization)
Is hydroquinone dangerous?
From an ecological point of view, hydroquinone, like all development products, is hazardous to ecosystems and especially to water, as it is not very biodegradable and partly toxic to fish. Once used, such as oil or used batteries, this product must be deposited in a specialized landfill in order not to pollute the environment. The developer can be heat treated in a suitable chemical facility.
There are no studies on the direct toxicity of hydroquinone in humans. However, several animal studies have shown that hydroquinone is toxic to the kidneys. In addition, hydroquinone is immunotoxic and is believed to play an important role in benzene immunotoxicity.
Experiments in rats have shown an increased incidence of certain tumors in the liver and kidneys after repeated administration of hydroquinone.
Therefore, hydroquinone, like other dihydroxybenzenes (for example, catechol), is believed to be carcinogenic and genotoxic.
-Possible mechanisms of action
In experiments with liver cells, hydroquinone led to a depletion of antioxidants in the cell, particularly glutathione. In addition, hydroquinone has been shown in cell culture to form DNA adducts and to increase the formation of 8-OHdG, a standard marker of DNA damage. This DNA damage is believed to be related to the formation of ROS.
-metabolism
In humans, hydroquinone is primarily metabolized to sulfate and glucuronide conjugates. In addition to reactive intermediates such as semiquinones and ROS, 1,4-benzoquinone is also formed in these metabolic pathways. This metabolism is catalyzed by various oxidases. Thus, the metabolism of hydroquinone is similar to that of catechol.
buy hydroquinone?
You can buy hydroquinone of the best quality at Laboratoriumdiscounter. Not only affordable but also available in different packaging and always with volume discount!
Technical data
Empirical formula C6H6O2
Molar mass (M) 110,11 g/mol
Density (D) 1,35
Boiling point (bp) 286 °C
Flash point (flp)165 °C • Melting point (mp)171-175 °C
Solubility 70 g/l (H2O, 25 °C)
ADR 9 III • WGK 3
CAS No.[123-31-9]
EG-Nr. 204-617-8 • UN-Nr. 3077
Downloads
$$$$$
Hazard Statements
H302 Harmful if swallowed
H317 May cause an allergic skin reaction
H318 Causes serious eye damage
H341 Suspected of causing genetic defects
H351 Suspected of causing cancer
H410 Very toxic to aquatic life with long lasting effects
Precautions - prevention
P273 Avoid release to the environment.
P280 Wear protective gloves/protective clothing/eye protection/face protection.
Precautions - response
P301+P312 IF SWALLOWED: Call a POISON CENTER/doctor if you feel unwell.
P302+P352 IF ON SKIN: Wash with plenty of soap and water.
P305+P351+P338 IF IN EYES: Rinse cautiously with water for an
amount of minutes; remove contact lenses, if possible; keep rinsing.
P333+P313 If skin irritation or rash occurs: Get medical advice/attention.