You have no items in your shopping cart
L (+) - Tartaric Acid ≥99.9 +% FCC, Ph. Eur, Foodgrade, E334
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Tartaric Acid?
This product is both food grade and pharmaceutical certified quality.
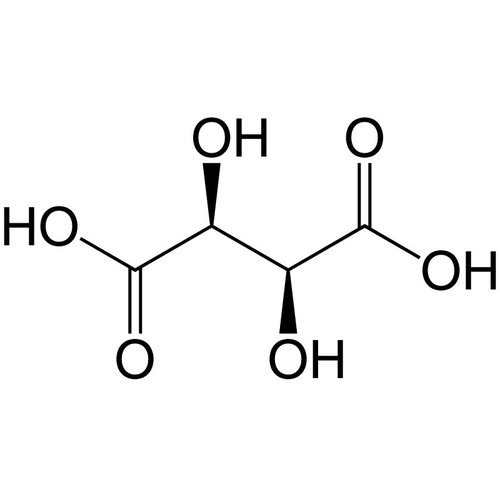
Tartaric acid, also known as 2,3-dihydroxysuccinic acid or 2,3-dihydroxybutanedioic acid in Latin as acidum tartaricum and in English as Tartaric acid, comes from the Greek tartaros inferno, because of its corrosive, burning effect.
It is a dicarboxylic acid in the α-hydroxycarboxylic acid group. It belongs to the sugar dicarboxylic acids (aldaric acids), their salts and esters are called tartrates. For example, L-(+)-tartaric acid occurs in grapes and is approved in the EU as a food additive E 334. In Germany, the total acidity of wines - calculated as tartaric acid - is given, although a number of other acids, especially malic acid, are present in wine.
Grape acid is the name of the racemate of tartaric acid. The intermolecular elimination of water produces the polymeric metatartaric acid, which is also used as a food additive under the name E 353.
L-(+)-tartaric acid and its calcium, potassium and magnesium salts are particularly abundant in the vines, grapes and leaves of the vine, as well as in dandelions, sugar beets, tamarinds, unripe rowan berries, in the seeds of the spindle tree, in the leaves of agaves, in black pepper, in pineapple and in many other fruits. During the winemaking process, poorly soluble salts of tartaric acid are deposited as crystals at the bottom of wine barrels or wine bottles. D-(-)-tartaric acid, erroneously called unnatural tartaric acid, is found only in the leaves of the West African orchid tree Bauhinia reticulata. The meso form does not exist in nature.
What is Tartaric Acid used for?
Only L-tartaric acid is more widely used, as it is the product of most tartaric acid synthesis processes. 50% of the L(+)-tartaric acid produced is used in the food and pharmaceutical industries, the other half in technical application areas.
Tartaric acid is used as an ingredient in disinfectants. As a rule, it is not specified whether it is L or D tartaric acid, the racemic mixed tartaric acid or another mixing ratio.
-Use as a food additive
The most obvious application of tartaric acid is its use as a food additive. L-tartaric acid, referred to in this area as E 334, not only occurs naturally in many foods, but is also added to many mixed food products for its flavor and preservative properties. Tartaric acid is used in the preparation of ice cream, artificial honey, fruits, lemonades and soft drinks, jellies, wine gums and confectionery, e.g. to stabilize creams and foams, and used to acidify low-acid wines. The term tartaric acid is also incorrectly used in baking books. The oral toxicity of L-tartaric acid was extremely low in rat animal studies; the oral LDLo for rats was 7500 mg/kg body weight.
Metatartaric acid (E 353), a polymer like lactone, is mainly used to stabilize tartaric acid; as a protective colloid, it prevents the crystallization of tartar in the wine.
-Technical use
Tartaric acid is also used in many technical areas, including to make silk feel better and smoother. The ability of tartaric acid to form complexes with metals is considerable: in these complexes the metal cation is more strongly bound by the tartaric acid than in most other organic acids. This results in numerous possible applications. For example, potassium sodium tartrate is used as a complexing agent in Fehling's solution, while tartaric acid is used to treat the surface of copper and brass items. The latter can also be used to clean floors contaminated with heavy metals, as it binds toxic heavy metals here, but is itself biodegradable. When added to cement and gypsum, it slows their setting by complexing the calcium ions and thus extends the working and formability time. It also serves as a reducing agent and for dissolving organic bases. In modern organic synthesis, LiAlH4 tartaric acid derivatives such as TADDOL are important chiral reagents or catalysts for the enantioselective reduction of ketones and other stereoselective synthesis processes.
Buy tartaric acid?
You can buy tartaric acid of both Foodgrade and Pharmaceutical quality at Laboratoriumdiscounter.nl Delivered quickly and for a friendly price! Available in different packaging and always with volume discount.
Technical information:
Empirical formula C4H6O6
Molar mass (M) 150.09 g / mol
Density (D) 1.76
Flash point (flp) 210 ° C • Melting point (mp) 166-169 ° C
Solubility 1390 g / l (H2O, 20 ° C)
WGK 1
CAS No. [87-69-4]
EC no. 201-766-0
Downloads
$$$$$
Hazard statements
H319 Causes serious eye irritation.
Safety recommendations
Precautions - response
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for a long period of time
amount of minutes; remove contact lenses, if possible; keep rinsing.