You have no items in your shopping cart
Tetrahydrofurane (THF) 99,8%
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Tetetrahydrofuran?
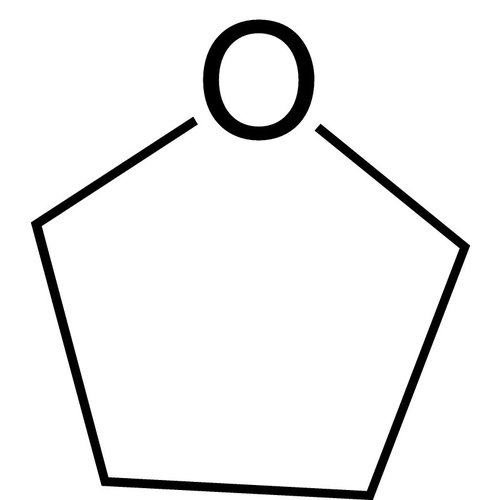
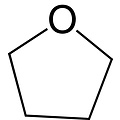
Tetrahydrofuran, 1,4-epoxybutane, oxolane, oxacyclopentane or even tetramethylene oxide, often more simply referred to as THF, is a heterocyclic organic compound. It is one of the most polar ethers and is used in organic synthesis as a solvent and as a precursor for the synthesis of polymers. It has a higher boiling point than most ethers (Teb = 66°C).
What is Tetrahydrofuran used for?
THF is a moderately polar, aprotic solvent. It is capable of resolving a wide variety of compounds.
Diethyl ether can often be substituted for THF, especially when a higher boiling point is required. Thus, THF, like diethyl ether, is often used in alkene hydroborations. Both ethers have an oxygen atom that can coordinate with an electrodedeficient boron atom, forming a Lewis acid-base adduct. Similarly, THF or diethyl ether are commonly used as solvents for Grignard reagents due to the ability of the oxygen atom to coordinate with the magnesium atom of the Grignard reagent (stabilizing it). Currently, THF can be replaced in organometallic reactions by 2-methyltetrahydrofuran (a neoteric solvent of renewable origin and benign for the environment), providing better results for Grignard reactions with benzyl or aryl reagents.
THF is often used in polymer science. For example, it can be used to dissolve rubber before determining its molecular mass by size exclusion chromatography.
THF is widely used industrially as a solvent for resins and plastics in dyes, paints, varnishes, adhesives and coatings, and in the food industry in the manufacture of food packaging.
Buy Tetrahydrofuran?
You can buy THF for a friendly price at Laboratoriumdiscounter.nl Fast delivery and available in different packaging. Always with volume discount and packaged in sturdy packaging. So order your Tetrahydrofuran at Laboratoriumdiscounter.nl
Technical information
Formula: C4H8O
MW: 72.105 g / mol
Boiling point: 66 ° C
Melting point: -108 ° C
Density: 0.886 g / cm³ (20 ° C)
CAS number: 109-99-9
Downloads
$$$$$
Hazard statements
H225 Highly flammable liquid and vapor
H302 Harmful if swallowed
H319 Causes serious eye irritation
H335 May cause respiratory irritation
H351 Suspected of causing cancer
Precautions - prevention
P210 Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. Do not smoke.
P280 Wear protective clothing / eye protection / face protection.
Precautions - response
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for a long period of time
amount of minutes; remove contact lenses, if possible; keep rinsing.
P308 + P313 NA (possible) exposure: Get medical attention.
Precautions - storage
P403 + P233 Store in a well-ventilated place. Keep container tightly closed.
Supplemental Hazard Information
EUH019 May form explosive peroxides.