You have no items in your shopping cart
Pyridine 99,75+% Extra pure
- Buy 2 and save 5%
- Buy 6 and save 10%
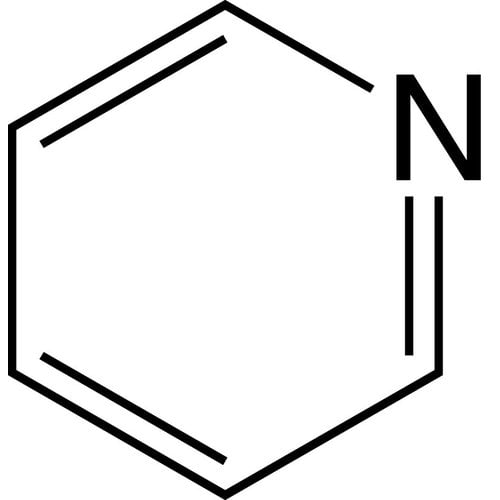
Pyridine or azine, with chemical formula C5H5N, is a simple and basic heterocyclic compound that approximates the structure of benzene, with one of the CH groups replaced by a nitrogen atom. It exists as a clear, slightly yellowish liquid with an unpleasant and pungent odor (acidic, rotten and reminiscent of fish). It is widely used in coordination chemistry as a ligand and in organic chemistry as a reagent and solvent. Pyridine derivatives are very numerous in pharmaceuticals and agrochemicals. Pyridine is used as a precursor in the manufacture of insecticides, herbicides, medicines, food flavors, dyes, adhesives, paints, and disinfectants. It is an aromatic compound that has a different reactivity than benzene.
Pyridine is a liquid under normal conditions of pressure and temperature. Pyridine is miscible with water and most common organic solvents. Pyridine is a medium polarity molecule, less polar than water and alcohols, but more polar than ethyl acetate, dichloromethane, petroleum ether and alkanes. In proton NMR, pyridine appears under three peaks21: 8.5 ppm for the -hydrogen of nitrogen, 7.6 ppm for the -hydrogen and 7.2 ppm in . In carbon NMR, pyridine is still below three peaks: at 150 ppm for carbon 1 and 5, 139 ppm for carbon 3, and 123 ppm for carbon 2 and 421. In infrared spectroscopy, pyridine shows an absorption band of about 3,000 cm−1 for the C-H of sp222 carbons. The olfactory detection threshold is 0.02 ppm (in air). The refractive index is 1.51024. The dielectric constant at 25°C is 12.425
Pyridine is commonly used as a reagent or catalyst in organic synthesis in condensation, dehalogenation, halogenation or acylation and also as a precursor for the synthesis of intermediates used in the manufacture of insecticides, herbicides, pharmaceuticals, food flavors, dyes, adhesives , paints, explosives and disinfectants. Pyridine is then used as a precursor to nucleophilic substitution reactions and more rarely electrophilic substitutions or alkylation reactions on nitrogen. Pyridine is also used to denature alcohol, antifreeze and fungicides, and also as an adjuvant for textile dyes.
Pyridine is often used as a polar basic solvent and helps neutralize acid formation in certain reactions. Pyridine is often used as a basic aprotic polar solvent or is simply added to the reaction medium to neutralize the acids resulting from these reactions. However, due to the high boiling temperature, pyridine is sometimes difficult to remove and other organic solvents with a low boiling temperature are used.
Deuterated d5-pyridine, in which the hydrogen atoms of pyridine have been replaced by deuterium atoms, can be used as a solvent in NMR spectroscopy.
Pyridine and its derivatives can be used to activate certain acylation or esterification reactions.
4-Dimethylaminopyridine (DMAP) is used to activate anhydrides during acylation reactions. The intermediate is a 1-acylpyridium salt that reacts with a primary or secondary amine to form an amide.
4-(1-pyrrolidinyl)-pyridine (PPY) activates an esterification reaction between a carboxylic acid and certain alcohols in the presence of DCC (dicyclohexylcarbodiimide). PPY reacts with the intermediate formed by the reaction between acid and DCC, which behaves similarly to an acid anhydride.
Pyridine is widely used as a ligand in coordination chemistry (in this context it is abbreviated as "py") because it has a great ability to form complexes with many transition metal cations. Pyridine is a fairly soft ligand in the HSAB theory. In complexes, a nitrogen-metal bond is formed. These complexes can be used for selective analyses.
Some of the pyridine complexes are used to oxidize primary or secondary alcohols.
Collins or Sarret's reagent consists of one equivalent of chromic acid and two equivalents of pyridine. They are prepared by heating and differ from each other in their crystalline form.
Conforth's reagent consists of one equivalent of chromium trioxide and two equivalents of pyridine mixed with water
PDC pyridinium dichromate consists of one dichromate equivalent of two equivalents of pyridine and hydrochloric acid
Pyridinium chlorochromate PCC consists of one equivalent of chromium trioxide, one equivalent of hydrochloric acid and pyridine.
Pyridine, with barbituric acid, is often used for the colorimetric detection of cyanides in aqueous solution. It reacts with cyanide chloride (formed by the reaction between cyanide ion and chloramine-T) to form a conjugated species with two molecules of barbituric acid, which together have a red hue. The color intensity is directly proportional to the cyanide concentration.
Technische gegevens:
Empirical formula C5H5N
Molar mass (M) 79,10 g/mol
Density (D) 0,98
Boiling point (bp) ca. 115 °C
Flash point (flp)17 °C • Melting point (mp)-42 °C
ADR 3 II • WGK 2
CAS No.[110-86-1]
EG-Nr. 203-809-9 • UN-Nr. 1282
Downloads
$$$$$
Hazard statements
H225 Highly flammable liquid and vapor
H302 + H312 + H332 Harmful if swallowed, in contact with skin or if inhaled
H315 Causes skin irritation
H319 Causes serious eye irritation
Precautions - prevention
P210 Keep away from open flames and hot surfaces. Do not smoke.
P233 Keep container tightly closed.
P280 Wear protective gloves / eye protection.
Precautions - response
P302 + P352 IF ON SKIN: Wash with plenty of water.
P304 + P340 IF INHALED: Remove person to fresh air and keep comfortable for breathing.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for a long period of time
amount of minutes; remove contact lenses, if possible; keep rinsing.