You have no items in your shopping cart
Copper(II) carbonate
Copper(II) carbonate or cupric carbonate is a chemical compound with formula CuCO
3. At ambient temperatures, it is an ionic solid (a salt) consisting of copper(II) cations Cu2+
and carbonate anions CO2−
3.
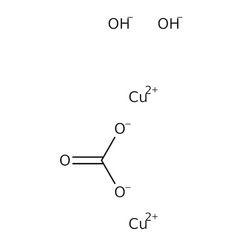
This compound is rarely encountered because it is difficult to prepare and readily reacts with water moisture from the air. The terms "copper carbonate", "copper(II) carbonate", and "cupric carbonate" almost always refer (even in chemistry texts) to a basic copper carbonate (or copper(II) carbonate hydroxide), such as Cu
2(OH)2CO
3 (which occurs naturally as the mineral malachite) or Cu
3(OH)2(CO
3)2 (azurite). For this reason, the qualifier neutral may be used instead of "basic" to refer specifically to CuCO
3.