You have no items in your shopping cart
Potassium persulfate
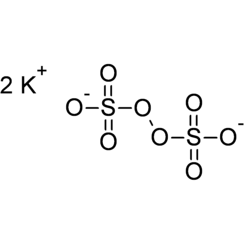
Potassium persulfate is the inorganic compound with the formula K2S2O8. Also known as potassium peroxydisulfate or KPS, it is a white solid that is sparingly soluble in cold water, but dissolves better in warm water. This salt is a powerful oxidant, commonly used to initiate polymerizations.
This salt is used to initiate polymerization of various alkenes leading to commercially important polymers such as styrene-butadiene rubber and polytetrafluoroethylene and related materials. In solution, the dianion dissociates to give radicals:
- [O3SO-OSO3]2− ⇌ 2 [SO4]•−
It is used in organic chemistry as an oxidizing agent, for instance in the Elbs persulfate oxidation of phenols and the Boyland–Sims oxidation of anilines.
As a strong yet stable bleaching agent it also finds use in various hair bleaches and lighteners. Such brief and non-continuous use is normally hazard free, however prolonged contact can cause skin irritation. It has been used as an improving agent for flour with the E number E922, although it is no longer approved for this use within the EU.