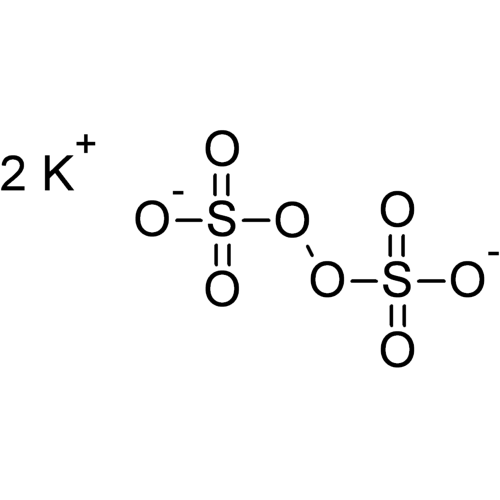You have no items in your shopping cart
Potassium persulfate 99+%
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Potassium Persulfate?
Potassium peroxodisulfate, often abbreviated to potassium persulfate or to KPS, is the potassium salt of peroxodisulfuric acid. The persulfate ion contains oxygen in the unstable −1 oxidation state. Therefore, potassium persulfate is a generator of free radicals and a very strong oxidizing or bleaching agent.
When potassium peroxodisulfate solutions are heated (70-100 °C) or exposed to UV radiation, the persulfate ion is broken down into sulfate radical anions. This reaction can be accelerated by suitable catalysts so that it takes place at considerably lower temperatures (20–50 °C). Examples of catalysts are metallic platinum, silver(I) and copper(II) ions.
Persulfates are among the strongest oxidizing agents, surpassed in their oxidizing power only by fluorine, ozone and oxygen fluorides. However, oxidation proceeds relatively slowly. They are able to oxidize almost all organic compounds. Iodine is slowly separated from iodide solutions, manganese(II) salt solutions are oxidized to manganese dioxide, and in the presence of catalytic silver(I) ions even to permanganate.
Potassium peroxodisulfate is stable as a crystalline solid, but decomposes at elevated temperatures with evolution of oxygen. At ~100 °C the decomposition is complete. In a moist or impure state, it tends to decompose even at significantly lower temperatures. Therefore, the connection must be protected from direct sunlight and other sources of heat during storage.
Solutions of potassium peroxodisulfate are acidic and decompose slowly at room temperature and rapidly at elevated temperatures. In a strongly acid environment (pH < 4) the decomposition takes place autocatalytically. The resulting acid catalyzes further decomposition of the persulfate ions.
Potassium peroxodisulfate can react violently with reducing agents. Mixtures of potassium peroxodisulphate and combustible substances can also burn in the absence of air.
What is Potassium Persulfate used for?
Potassium peroxodisulfate is a commonly used initiator for emulsion and solution polymerization, for example in the manufacture of polyacrylates, polyvinyl acetate and polyvinyl chloride and in the emulsion copolymerization of acrylonitrile, 1,3-butadiene, styrene and other monomers. These reactions with an amount of usually 0.1-0.5% potassium peroxodisulfate are usually carried out at 75 to 95°C. Polymerizations at lower temperatures are also possible in combination with redox systems. In cosmetics, potassium peroxodisulfate is used as an essential component of bleaching agents for hair bleaching and as a bleaching agent in hair dyes. Potassium peroxodisulfate is used in textile finishing as a de-sizing agent and as a bleach activator, especially in cold bleaching processes. It is also used as an oxidizing agent in chemical synthesis (e.g. Elbs oxidation of phenols to p-diphenols in alkaline solution) and in analyses, e.g. as a digesting agent.
Potassium peroxodisulfate is also used in laboratories to clean glassware, in papermaking to modify starch.
In the metal and electronics industry, potassium peroxodisulfate is used to a small extent for blackening brass (process according to Erich Groschuff) and for etching printed circuit boards. In the latter case, the surface is either cleaned by oxidation (etching) or the copper is removed by oxidation (etching) However, due to its higher water solubility, sodium persulfate is preferred for this application.
In analog photography, potassium persulfate can be used as a reducing agent to remove traces of thiosulfate from negatives during film processing. In the past, potassium peroxodisulfate was also used as a flour improver for "flour improvement". This has been banned in Europe since 1956/57.
Buy potassium persulfate?
Buy potassium persulphate of the highest quality at Laboratoriumdiscounter.nl. Potassium persulfate for a friendly price and delivered quickly. Always with volume discount and available in different packaging.
What Safety Precautions Should I Take Before Using Chemicals?
It is always wise to work as safely as possible when working with chemicals. Even substances that do not seem dangerous at first can cause enormous damage if they get into your eyes. Therefore, always wear safety goggles. You also want to prevent chemicals from ending up on your skin, which is why it is important to always use good gloves or disposable gloves.
Respiratory protection is necessary for volatile substances, vaporous liquids and solids that dust. There are many different types of filters, so you should always refer to the MSDS to find out which filter you need. Many filters also specify which substances they are for.
In some cases it may be necessary to protect the whole body, in which case a plastic overall is needed, you can find it here.
When working with flammable and oxidizing substances, it is important to always have fire extinguishers and absorbents at hand. It is also useful to be able to clean up spills of aggressive chemicals immediately with absorbents and to dispose of them in accordance with international and/or local legislation.
If you need more information on how to handle a specific substance, always consult the safety data sheet. You can find this on the product page or request it via [email protected]
Technical data
Potassium peroxodisulphate
Empirical formula K2S2O8
Molar mass (M) 270.32 g/mol
Density (D) 2.48 g/cm³
Melting point (mp) 100°C
ADR 5.1 III
WCK 1
CAS no. [7727-21-1]
EC-No. 231-781-8
UN-No. 1492
Downloads