You have no items in your shopping cart
Sodium hypochlorite
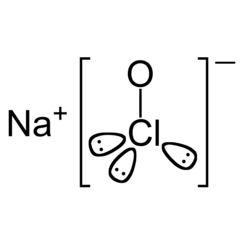
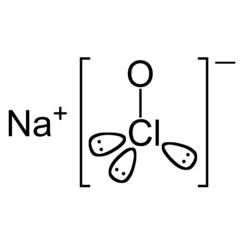
Sodium hypochlorite is a chemical compound with the formula NaOCl or NaClO, comprising a sodium cation (Na+) and a hypochlorite anion (OCl−or ClO−). It may also be viewed as the sodium salt of hypochlorous acid. The anhydrous compound is unstable and may decompose explosively. It can be crystallized as a pentahydrate NaOCl·5H
2O, a pale greenish-yellow solid which is not explosive and is stable if kept refrigerated.
Sodium hypochlorite is most often encountered as a pale greenish-yellow dilute solution commonly known as liquid bleach or simply bleach, a household chemical widely used (since the 18th century) as a disinfectant or a bleaching agent. The compound in solution is unstable and easily decomposes, liberating chlorine, which is the active principle of such products. Indeed, sodium hypochlorite is the oldest and still most important chlorine-based bleach.
Its corrosive properties, common availability, and reaction products make it a significant safety risk. In particular, mixing liquid bleach with other cleaning products, such as acids or ammonia, may produce toxic fumes.