You have no items in your shopping cart
Diethylene glycol 99 +% Extra pure
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Diethylene Glycol?
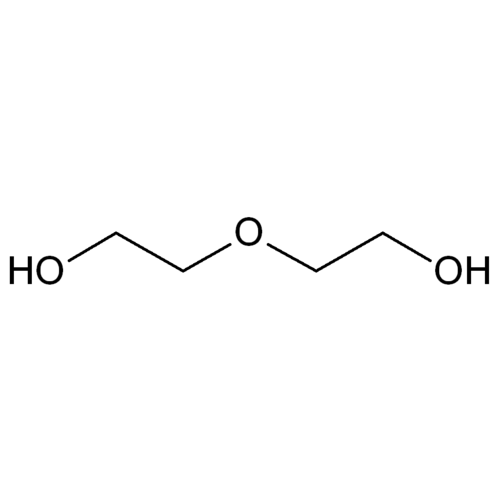
Diethylene glycol is a derivative of ethylene glycol and belongs to the group of alcohols (diols) or, more precisely, glycol ethers.
Diethylene glycol is synthesized by the ethoxylation of ethylene glycol with ethylene oxide. It is usually a by-product of ethylene glycol production.
Diethylene glycol forms flammable vapour-air mixtures at high temperatures. The compound has a flash point of 138 °C. The explosion range is between 1.7 volume percent (75 g/m3) as lower explosive limit (LEL) and 37 volume percent (1635 g/m3) as upper explosive limit (UEL). The ignition temperature is 355 ° C. The substance therefore falls into temperature class T2.
What is Diethylene glycol used for?
Most of the diethylene glycol produced is used as a raw material for the synthesis of polyester resins, morpholine and 1,4-dioxane. It is rarely used as a solvent for nitrocellulose, synthetic resins, dyes, oils and some other organic substances. In addition, diethylene glycol is rarely used as a wetting agent for corks, inks and glues; According to a statement from the Federal Institute for Risk Assessment (BfR), use in the context of food or, for example, in toothpaste should be restricted.
A mixture of diethylene glycol and water can be used as an antifreeze because the mixture has a lower melting point than pure water. As a rule, however, the more suitable ethylene glycol - the main component of BASF's "Glysantin" refrigerant - is used. However, in a diethylene glycol-water mixture, not only is the melting point lowered, the boiling point is also increased more than in the water-ethylene glycol system. Diethylene glycol can therefore also be used as an additive in hydraulic and brake fluids.
Buy Diethylene glycol?
If you need diethylene glycol of the highest quality, you have come to the right place at Laboratory Discounter. Not only is it delivered quickly but also available in different packaging. Always with volume discount!
Technical data:
2,2'-Dihydroxy diethyl ether, Diglycol
Empirical formula C4H10O3
Molar mass (M) 106.12 g / mol
Density (D) 1.12 g / cm³
Boiling point (bp) 252 ° C
Flash point (flp) 138 ° C
Melting point (mp) -6.5 ° C
WGK 1
CAS No. [111-46-6]
EC no. 203-872-2
$$$$$
Hazard statements
H302 Harmful if swallowed
H373 May cause damage to organs (kidney) through prolonged or repeated exposure
Precautions - prevention
P260 Do not breathe mist / vapor.
P270 Do not eat, drink or smoke when using this product.
Precautions - response
P301 + P312NA INGESTION: Call a POISON CENTER or doctor if you feel unwell
%%%%%
| MSDS Di-ethyleenglycol (NL) |
| MSDS Diethylenglykol (DE) |
| MSDS Diethylene glycol(EN) |
| MSDS Diéthylene glycol (FR) |
| MSDS Dietilenglicol (ES) |