You have no items in your shopping cart
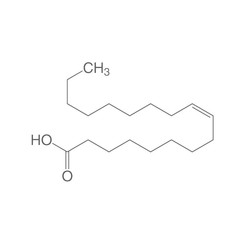
Oleic acid
Oleic acid, also called oleic, is the main representative of the monounsaturated fatty acids (18: 1). Its systematic name is (Z) -9 octadecenoic acid and it is an omega-9 fatty acid due to the location of its double bond. A naturally occurring isomer of oleic acid is petroselic acid, the trans isomer is known as elaidic acid. The salts and esters of oleic acid are called oleates.
The fatty acids can be obtained from the corresponding triacylglycerides by alkaline saponification by boiling the corresponding fats or oils with bases. The saponification itself initially supplies their salts. The free fatty acids are obtained by neutralization with (mineral) acids. Since natural fats and oils always contain many different fatty acids, the resulting mixture is usually separated by distillation.
Commercially, oleic acid is obtained from tallow by hydrolysis and subsequent crystallization. This process can yield concentrations of about 70%, also known as olein. In addition to oleic acid, this mixture may contain palmitoleic acid, linoleic acid and other unsaturated and saturated fatty acids. By hydrolyzing vegetable oils very rich in oleic acid, such as olive oil, Euphorbia lathyris or high oleic sunflowers, purities in excess of 90% can be obtained.
Oleic acid is used as a component of mixtures with other fatty acids in variable proportions, mainly for the manufacture of soaps and for the production of surfactants. Its use as a lubricant in the textile industry has been proven.