You have no items in your shopping cart
Diethylene glycol
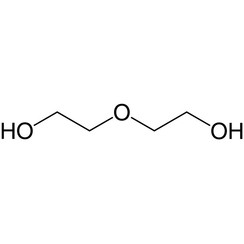
Diethylene glycol (DEG) is an organic compound with the formula (HOCH2CH2)2O. It is a colorless, practically odorless, poisonous, and hygroscopic liquid with a sweetish taste. It is miscible in water, alcohol, ether, acetone, and ethylene glycol. DEG is a widely used solvent. It can be a contaminant in consumer products; this has resulted in numerous epidemics of poisoning since the early 20th century.
Diethylene glycol is used in the manufacture of saturated and unsaturated polyester resins, polyurethanes, and plasticizers. DEG is used as a building block in organic synthesis, e.g. of morpholine and 1,4-dioxane. It is a solvent for nitrocellulose, resins, dyes, oils, and other organic compounds. It is a humectant for tobacco, cork, printing ink, and glue.[6] It is also a component in brake fluid, lubricants, wallpaper strippers, artificial fog and haze solutions, and heating/cooking fuel.[1] In personal care products (e.g. skin cream and lotions, deodorants), DEG is often replaced by selected diethylene glycol ethers. A dilute solution of diethylene glycol can also be used as a cryoprotectant; however, ethylene glycol is much more commonly used. Most ethylene glycol antifreeze contains a few percent diethylene glycol, present as an byproduct of ethylene glycol production.