You have no items in your shopping cart
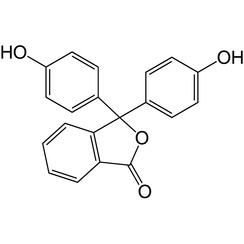
Phenolphthalein
Phenolphthalein is a chemical compound with the formula C20H14O4 and is often written as "HIn" or "phph" in shorthand notation. Phenolphthalein is often used as an indicator in acid–base titrations. For this application, it turns colorless in acidic solutions and pink in basic solutions. It belongs to the class of dyes known as phthalein dyes.
Phenolphthalein is slightly soluble in water and usually is dissolved in alcohols for use in experiments. It is a weak acid, which can lose H+ ions in solution. The phenolphthalein molecule is colorless, and the phenolphthalein ion is pink. When a base is added to the phenolphthalein, the equilibrium shifts, leading to more ionization as H+ ions are removed. This is predicted by Le Chatelier's principle