You have no items in your shopping cart
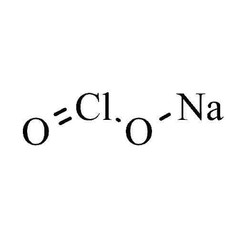
Sodium chlorite
Sodium chlorite (NaClO2) is a chemical compound used in the manufacturing of paper and as a disinfectant.
Use
The main application of sodium chlorite is the generation of chlorine dioxide for bleaching and stripping of textiles, pulp, and paper. It is also used for disinfection of municipal water treatment plants after conversion to chlorine dioxide. An advantage in this application, as compared to the more commonly used chlorine, is that trihalomethanes (such as chloroform) are not produced from organic contaminants. Chlorine dioxide generated from sodium chlorite is approved by FDA under some conditions for disinfecting water used to wash fruits, vegetables, and poultry.
Sodium chlorite, NaClO2, sometimes in combination with zinc chloride, also finds application as a component in therapeutic rinses, mouthwashes, toothpastes and gels, mouth sprays, as preservative in eye drops, and in contact lens cleaning solution under the trade name Purite.
It is also used for sanitizing air ducts and HVAC/R systems and animal containment areas (walls, floors, and other surfaces).
Chemical reagent
In organic synthesis, sodium chlorite is frequently used as a reagent in the Pinnick oxidation for the oxidation of aldehydes to carboxylic acids. The reaction is usually performed in monosodium phosphate buffered solution in the presence of a chlorine scavenger (usually 2-methyl-2-butene).
In 2005, sodium chlorite was used as an oxidizing agent to convert alkyl furans to the corresponding 4-oxo-2-alkenoic acids in a simple one pot synthesis.
Acidified sodium chlorite
Mixing sodium chlorite solution with a weak food-grade acid solution (commonly citric acid), both stable, produces short-lived acidified sodium chlorite (ASC) which has potent decontaminating properties. Upon mixing the main active ingredient, chlorous acid is produced in equilibrium with chlorite anion. The proportion varies with pH, temperature, and other factors, ranging from approximately 5–35% chlorous acid with 65–95% chlorite; more acidic solutions result in a higher proportion of chlorous acid. Chlorous acid breaks down to chlorine dioxide which in turn breaks down to chlorite anion and ultimately chloride anion. Sodium chlorite is used for sanitation of the hard surfaces which come in contact with food and as a wash or rinse for a variety of foods including red meat, poultry, seafood, fruits and vegetables. Because the oxo-chlorine compounds are unstable when properly prepared, there should be no measurable residue on food if treated appropriately.Sodium chlorite also is used as a teat dip for control of mastitis in dairy cattle.
Use in public crises
The U.S. Army Natick Soldier Research, Development, and Engineering Center produced a portable "no power required" method of generating chlorine dioxide, known as ClO2, gas, described as one of the best biocides available for combating contaminants, which range from benign microbes and food pathogens to Category A Bioterror agents. In the weeks after the 9/11 attacks when anthrax was sent in letters to public officials, hazardous materials teams used ClO2 to decontaminate the Hart Senate Office Building, and the Brentwood Postal Facility.
In addressing the COVID-19 pandemic, the U.S. Environmental Protection Agency has posted a list of many disinfectants that meet its criteria for use in environmental measures against the causative coronavirus. Some are based on sodium chlorite that is activated into chlorine dioxide, though differing formulations are used in each product. Many other products on the EPA list contain sodium hypochlorite, which is similar in name but should not be confused with sodium chlorite because they have very different modes of chemical action.