You have no items in your shopping cart
Calcium hypochlorite 65%
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Calcium Chloride?
Calcium hypochlorite is the calcium salt of hypochlorous acid with the chemical formula Ca(OCl)2. It should be distinguished from the so-called chlorinated lime, which is a technical mixture of calcium chloride, calcium hypochlorite and calcium hydroxide resulting from the manufacturing process.
Calcium hypochlorite is a white powder or granulate that smells like chlorine. It is sparingly soluble in water; concentrated solutions are greenish-yellow in color.
What is Calcium Hypochlorite used for?
In technology, chlorinated lime and calcium hypochlorite are used as a bleaching agent for cellulose, paper and textiles, but for environmental reasons they are being replaced by other chlorine-free bleaching processes. Calcium hypochlorite is also used for the production of chloroform, for the bleaching of shellac, and for the extraction of cobalt for the separation of other metals. It is also used for water treatment in swimming pools for public and private swimming pools.
-safety instructions
Calcium hypochlorite is harmful to health and should be stored in a cool and dry place as it tends to decompose and heat itself up. Oxidizable substances and acids should be kept away.
-Technical information:
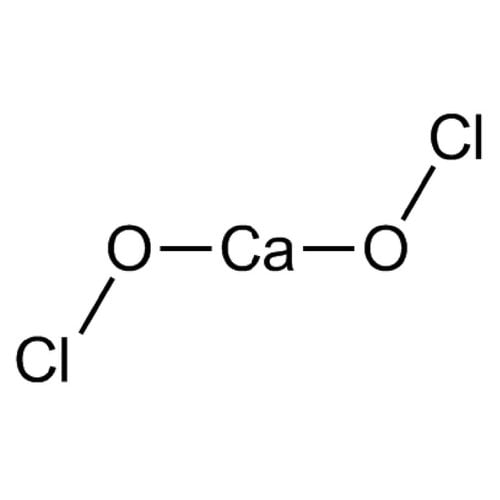
Empirical formula CaCl2O2
Molar mass (M) 142.99 g/mol
Density 2.35
Solubility: 200 g/l (H2O, 20 °C)
ADR 5.1 II • WGK 3
CAS No.[7778-54-3]
EC-No. 231-908-7 • UN No. 1748
$$$$$
Hazard statements
H272 May intensify fire; oxidizing
H302 Harmful if swallowed
H314 Causes severe burns and eye damage
H410 Very toxic to aquatic life with long lasting effects
Safety recommendations
Precautions - prevention
P210 Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. Do not smoke.
P280 Wear protective gloves / eye protection.
Precautions - response
P303 + P361 + P353 IF ON SKIN (or hair): Immediately contaminate clothing
pull out. Rinse skin with water [or shower].
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for a long period of time
amount of minutes; remove contact lenses, if possible; keep rinsing.
P310 Immediately call a POISON CENTER / doctor / ....
P370 + P378 In case of fire: Use sand for extinction - never use water.
Supplemental Hazard Information
EUH031 Contact with acids liberates toxic gas.




%%%%%
| MSDS Calciumhypochloriet (NL) |
| MSDS Calciumhypochlorid (DE) |
| MSDS Calcium hypochlorite (EN) |
| MSDS Hypochloriet de calcium (FR) |
| MSDS Hipoclorito de calcio (ES) |