You have no items in your shopping cart
Benzoic Acid ≥99.98%, Ph.Eur., USP, BP, Foodgrade, E210
- Buy 2 and save 5%
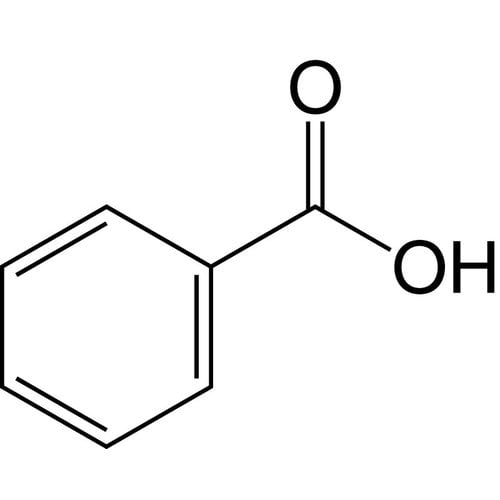
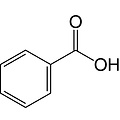
Benzoic acid is an aromatic carboxylic acid. It consists of a phenyl radical with a carboxy group. The salts and esters of benzoic acid are called benzoates.
Benzoic acid is used in the production of benzoic acid esters, which are used in the perfume industry as fragrances (such as ethyl benzoate) or as biocides (e.g. benzyl benzoate). Benzoic acid is also used for plasticizers in the preparation of benzoyl compounds such as benzoyl chloride and dibenzoyl peroxide.
In the food industry, benzoic acid (E210) is often used as a preservative in shelf-stable foods such as ketchup, mustard and other sauces, as well as in sausages, margarine, fish salads and many other products, but especially in pickled foods. Due to their better solubility, the salts are more often used: sodium benzoate (E 211), potassium benzoate (E 212), calcium benzoate (E 213). In addition, benzoic acid is approved in the European Union as an additive for finishing pigs.
Furthermore, benzoic acid is often used as a preservative in tobacco products.
Benzoic acid is used for the treatment of skin fungi and is approved for the preservation of cosmetics in accordance with the German Cosmetics Regulation. The bacteriostatic and fungistatic action is based on the inhibitory action on enzymes that degrade reactive oxygen species (catalase and peroxidase), causing an accumulation of hydrogen peroxide in the cells of the microorganisms. This eventually leads to their death.
In environmental monitoring, bottom traps are filled with a saturated benzoic acid solution to kill trapped organisms such as insects or snails and hold them in place until the next emptying.
Benzoic acid is a basic titer according to the Pharmacopoeia.
Empirical formula C7H6O2
Molar mass (M) 122.12 g / mol
Density (D) 1.321
Boiling point (bp) 249 ° C
Flash point (flp) 121 ° C • Melting point (mp) 121-123 ° C
Solubility 2.9 g / l (H2O, 20 ° C)
WGK 1
CAS No. [65-85-0]
EC no. 200-618-2
$$$$$
Hazard statements
H315 Causes skin irritation
H318 Causes serious eye damage
H372 Causes damage to organs (lung) through prolonged or repeated exposure (after inhalation)
Safety recommendations
Precautions - prevention
P260 Do not breathe dust.
P280 Wear protective gloves / eye protection.
Precautions - response
P302 + P352 IF ON SKIN: Wash with plenty of water.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for a long period of time
amount of minutes; remove contact lenses, if possible; keep rinsing.
P310 Immediately call a POISON CENTER / doctor.