You have no items in your shopping cart
Hexamethylenetetramine (Hexamine) ≥99.5%, extra pure
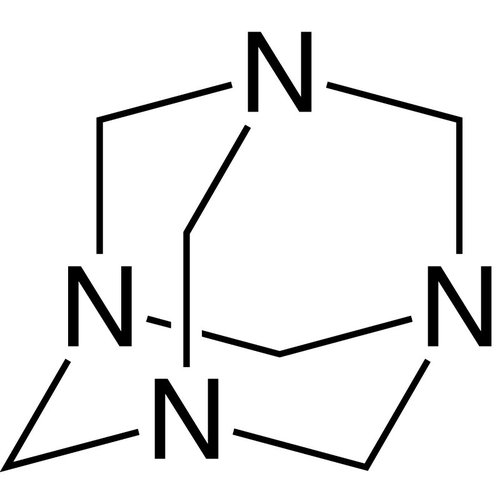
Hexamethylenetetramine (hexamine, methenamine or urotropine) is a colorless crystalline powder. The chemical structure of hexamethylenetetramine can be derived from the hydrocarbon adamantane: In hexamethylenetetramine, nitrogen atoms are located at the four connection points of the three six-membered rings. There is a CH2 group between the nitrogen seam (like the vertices of a tetrahedron).
Hexamethylenetetramine was first described by Alexander Michailowitsch Butlerow in 1859. In 1894 it was introduced into therapy under the trademark Urotropin for disinfecting the urinary tract.
The flash point is 250°C, the ignition temperature 390°C and the decomposition temperature > 263°C. The energy density, calorific value is 31.3 MJ/kg, the pH value is 8.4 at 28 g/l and the pKa value is 8.95 (20 °C)
Hexamethylenetetramine is used in the production of amino and phenoplasts. In pressed form, it is also used as a dry fuel and is the main component of Esbit fuel - in the form of cube-shaped tablets with gray markings.
In histochemistry, hexamethylenetetramine is used for silver staining.
In organic synthesis, it serves as formyl equivalent (Duff reaction), for the introduction of amino groups, for the synthesis of N-heterocycles, and is used in the Mannich reaction. In inorganic analysis it is used in the cation separation process as a buffering agent for the precipitation of the "urotropin group" (which also includes iron, chromium and aluminum) at pH 5.5. In acidic aqueous solution, methenamine is broken down to formaldehyde and ammonium ions. For this reason, sublimated hexamethylenetetramine leads to excessive formaldehyde concentrations when measuring emissions during the production of phenolic resins in measuring processes that work with acidic solutions. In this regard, the DNPH method and the MBTH method should be mentioned.
In foundry technology, it is used together with phenolic resin as a resin hardener system for the mask molding process for the production of mold shells and hollow cores.
Another application is the neutralization of acid by-products in the synthesis of the chemical warfare agent sarin, so the detection of hexamine (=hexamethylenetetramine) in combat areas can be regarded as an indication for the use of sarin.
Empirical formula C6H12N4
Molar mass (M) 140,19 g/mol
Density (D) 1,33
Flash point (flp)250 °C • Melting point (mp)ca. 263 °C (subl.)
Solubility 895 g/l (H2O, 20 °C)
ADR 4.1 III • WGK 1
CAS No.[100-97-0]
EG-Nr. 202-905-8 • UN-Nr. 1328
$$$$$
Hazard statements
H228 Flammable solid
H317 May cause an allergic skin reaction
Precautions - prevention
P210 Keep away from heat. Do not smoke.
Precautions - response
P333 + P313 In case of skin irritation or rash: Get medical attention.