You have no items in your shopping cart
Vanillin 99,5+% Extra Pure, Foodgrade
- Buy 2 and save 5%
- Buy 6 and save 10%
 Vanillin is a natural aromatic aldehyde that develops in vanilla beans when they are prepared as a spice.
Vanillin is a natural aromatic aldehyde that develops in vanilla beans when they are prepared as a spice.
It is used to make natural flavors.
Of the many components of vanilla's natural aroma, vanillin is the most important and characteristic. It represents 0.75% to 2% of the pod's mass. A pod of about three grams contains only 22 to 60 mg.
It was first extracted in its pure state by the chemist Théodore Nicolas Gobley by maceration of the vanilla in alcohol at 85°, followed by an extraction with ether. The very fragrant brown substance it obtains after evaporation is boiled in water and then filtered hot. Vanillin is finally isolated after several successive recrystallizations in the form of long colorless needles.
Vanillin was first synthesized in 1874 by Wilhelm Haarmann and Ferdinand Tiemann, from coniferin, an isoeugenol derivative found in pine bark. Karl Reimer proposed a new synthetic route from guaiacol two years later in 1876.
The molecule is an aromatic aldehyde, hence the other names of vanillaldehyde or vanillin aldehyde. In solution, in the presence of iron and another alkaline compound, the aldehyde takes on a red color and loses its odorant power.
Vanillin has a similar smell to vanilla with a sweet taste. However, its aromatic intensity is two to four times less potent than that of ethyl vanillin.
Vanillin can be made cheaply in several ways, while vanilla is very expensive to produce and prepare. For example, the industrial production of vanillin and its use in food and perfumery has become much more important than the production and use of natural vanilla.
-Usage
Vanillin is used for its flavoring properties, alone or as part of a flavoring agent. It should not be abused in the aroma because it has a bitter taste in high doses. Vanillin (Fema GRAS number 31079) is used in making vanilla, chocolate and banana flavours.
It is an intermediate for the production of various derivatives for pharmaceutical use.
Due to its chemical properties, it is sometimes used in certain reactions in analytical chemistry, especially in the form of sulfovanillin.
Aphrodisiac properties are also attributed to it
Sulfuric acid vanillin (mixture in concentrated sulfuric acid) is used to dose terpenes by colorimetry
-Buy Vanillin from Laboratory Discounter
Buying vanillin at Laboratoriumdiscounter is easy and you are guaranteed the best quality. In addition, the Vanillin from Laboratory Discounter is certified food grade! All that for a nice price, so beautiful and in the case of Vanilline very tasty!
-Technical data:
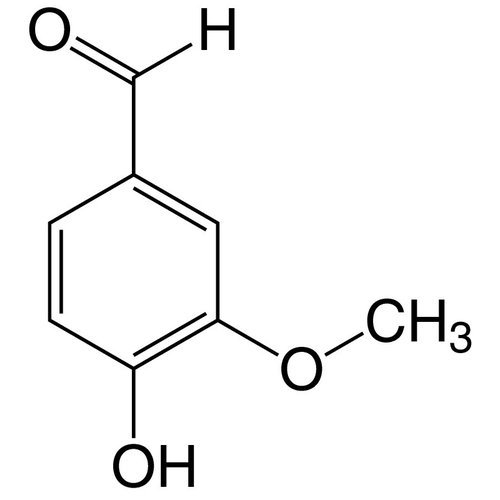
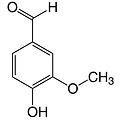
4-Hydroxy-3-methoxy-benzaldehyde
Empirical formula C8H8O3
Molar mass (M) 152.15 g/mol
Density (D) 1.06 g/cm³
Boiling point (bp) 285 °C
Flash point (flp) 160.8°C
Melting point (mp) 83°C
WCK 1
CAS no. [121-33-5]
EC-No. 204-465-2
$$$$$
hazard statements
H319
Warning
Causes serious eye irritation
Precautionary statements
Precautionary statements - prevention
P280 Wear protective gloves/eye protection Precautionary statements - response
P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuous ringing
P337+P313 If eye irritation persists: Get medical advice/attention