You have no items in your shopping cart
Ammonium Carbonate 30.5+% NH3, Food Grade FCC
- Buy 2 and save 5%
What is Ammonium Carbonate?
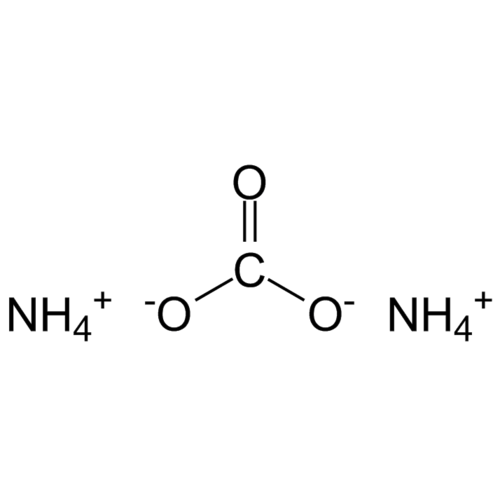
Ammonium carbonate, (NH4)2CO3 H2O, forms water-soluble, colorless, cubic crystals. From 58 °C it completely decomposes into ammonia, carbon dioxide and water.
The salt forms a colorless crystal with a faint smell of ammonia. It has a density of about 1.6 g cm−3 (at 20 °C). In aqueous solution, this salt, which has a weak acid as cation and a weak base as anion, is somewhat basic. The pH value is the result of a complex equilibrium that develops between dissolved ammonia NH3, ammonium ions NH4+, carbonate ions CO32−, hydrogen carbonate ions HCO3− and carbon dioxide CO2 as a result of salt hydrolysis. The pH of a 10% aqueous solution of ammonium carbonate is 9.4.
The ammonium carbonate salt reacts to hydrogen carbonate and hydroxide ions during dissolution in water. An aqueous solution of ammonium carbonate is therefore only stable in a neutral and weakly basic environment - when strong acids are added gaseous carbon dioxide escapes, when concentrated alkalis are added ammonia gas escapes.
When dry, ammonium carbonate decomposes slowly in air, but decomposes violently when heated.
What is Ammonium Carbonate Used For?
Ammonium carbonate is used in the synthesis of heterocycles and as an additive in photographic developers. It is also used in dyeing as a stain, as a staining agent, and as a carbon dioxide generator in fire extinguishers.
It is also used as a leavening agent (as a component of staghorn salt). In the EU it is approved as a food additive with number E 503i.
It was also used in the past as a smelling salt to stimulate dizziness and fainting.
Ammonium carbonate is commonly used in inorganic chemistry for a qualitative analysis to separate the alkaline earth metal cations of barium, strontium and calcium as a group from an unknown sample in the cation separation process and identify them using detection reactions.
It is also used to make catalysts, foams, hair treatment products, and casein paints and adhesives.
Buy ammonium carbonate?
This Ammonium carbonate is of the highest quality and also a food grade! It is therefore suitable to be used as a food additive. So do you need ammonium carbonate? Then order quickly at Laboratoriumdiscounter. Friendly priced and delivered quickly!
Technical information
≥30.5 %, NH3, extra pure
Empirical formula CH6N2O2 CH5NO3 (1:1)
Molar mass (M) 157.13 g/mol
Solubility: approx. 320 g/l (H2O, 20 °C)
WCK 1
CAS No.[10361-29-2]
EC-No. 233-786-0
Downloads
$$$$$
Hazard Statements
H302 Harmful if swallowed
H315 Causes skin irritation
H318 Causes serious eye damage
Safety Recommendations
Precautions - prevention
P270 Do not eat, drink or smoke when using this product.
P280 Wear protective gloves/eye protection.
Precautions - response
P305+P351+P338 IF IN EYES: Rinse cautiously with water for an
amount of minutes; remove contact lenses, if possible; keep rinsing.
P310 Immediately call a POISON CENTER/doctor.
Hazardous ingredients for labelling: Ammonium carbamate, Ammonium hydrogen carbonate