You have no items in your shopping cart
Butyric acid
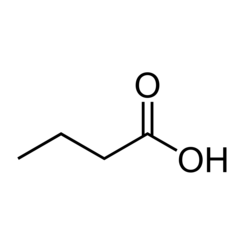
Butyric acid, also known by the systematic name butanoic acid, is a carboxylic acid with the structural formula CH3CH2CH2CO2H. Classified as a carboxylic acid, it is an oily, colorless liquid that is soluble in water, ethanol and ether. Isobutyric acid (2-methylpropanoic acid) is an isomer. Butyric acid salts and esters are known as butyrates or butanoates. The acid is not common in nature, but the esters are widespread. It is a very common industrial chemical.
Butyric acid is used in the preparation of various butyrate esters. It is used to produce cellulose acetate butyrate (CAB), which is used in a wide variety of tools, parts and coatings, and is more resistant to degradation than cellulose acetate. However, CAB can break down through exposure to heat and moisture, releasing butyric acid.
Low molecular weight esters of butyric acid, such as methyl butyrate, usually have pleasant aromas or flavors. As a result, they are used as food and perfume additives. It is an approved nutritional flavor in the EU FLAVIS database (number 08.005).
Due to its powerful fragrance, it has also been used as an additive to fishing bait. Many of the commercially available flavors used in carp (Cyprinus carpio) bait use butyric acid as the ester base; however, it is not clear whether fish are attracted to the butyric acid itself or the substances added to it. Butyric acid, however, was one of the few organic acids shown to be palatable to both tench and bitterness. The substance has also been used by the Sea Shepherd Conservation Society as a stench bomb to disrupt Japanese whalers.