You have no items in your shopping cart
Butyric Acid 99.5+% Extra Pure
- Buy 2 and save 5%
- Buy 6 and save 10%
What is butyric acid?
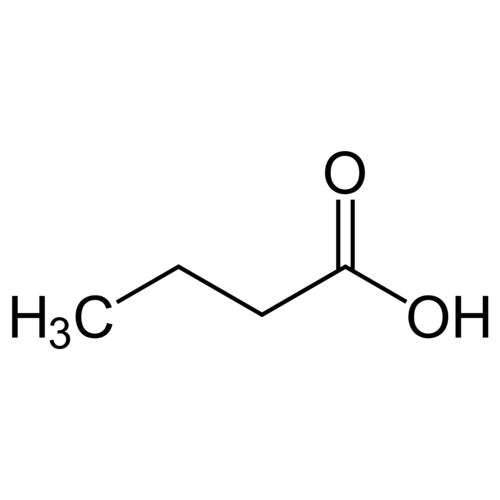
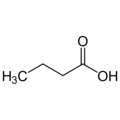
Butanoic acid, also called butyric acid from the Greek βουτυρος (butter), is a saturated carboxylic acid with the formula CH3CH2CH2-COOH.
For example, it occurs in rancid butter, parmesan cheese and gastric juices, where it gives off a strong and unpleasant odor. Butyric acid can be detected from about 5 to 40 µg/m3, depending on the sources.
Under normal conditions of temperature and pressure, butanoic acid is a slightly oily liquid that solidifies at -8°C and has a boiling point of 164°C. It is easily soluble in water, ethanol and ether and dissociates from the solvent upon addition of calcium chloride. Potassium dichromate and sulfuric acid oxidize it to carbon dioxide and acetic acid, while the alkaline potassium permanganate only oxidizes it to carbon dioxide. In addition, its isomer of composition is 2-methylpropanoic acid, which gets its second name isobutyric acid.
It is also a short-chain fatty acid found in vegetable oils and animal fats. The glyceride (glycerol ester) of butyric acid makes up 3% to 4% of the butter. When butter goes rancid, the glycerides are hydrolyzed, releasing the foul-smelling butyric acid. Normal butyric acid or fermentation butyric acid is also found as a hexyl ester in the oil of Heracleum giganteum and as an octyl ester in that of parsnip (Pastinaca sativa); it is also present in perspiration.
It is usually produced by the fermentation of sugar or starch, caused by the addition of decomposing cheese, to which calcium carbonate is added to neutralize the acids formed during the process. The butyric acid fermentation of starch is facilitated by the direct addition of Bacillus subtilis.
Various esters are obtained from butyric acid. These esters are called butyrate or more precisely butanoate. Those with a low molar mass, such as methyl butanoate, usually have pleasant aromas. So they are used as food additives or in perfumes.
The molecular formula is the same as that of propyl formate.
What is butyric acid used for?
Butanoic acid is used in the preparation of various flavorings (butanoate esters), low molecular weight butyric acid esters such as methyl butanoate, for the most part, have pleasant aromas or flavors. Because of this, they are used as additives in food and perfumes.
Thanks to its strong smell, it is also used as an additive for fishing baits. Many flavors available on the market to lure carp (Cyprinus carpio) use butyric acid as an ester base, but it is unclear whether fish are attracted to butyric acid itself or the other added substances. However, butyric acid is one of the few organic acids that has proven palatable to both tench and roach.
Anecdotally, this substance has also been used as a non-toxic, nauseating, flesh-contaminating repellent by the Sea Shepherd team against the Japanese whaling factory ship and by anti-abortion activists; some police departments are also considering using it as a non-lethal weapon.
In addition to hydrogen sulfide, butyric acid is used to produce cheap, highly effective and durable stink bombs.
The pungent fragrance is also used to drive away moles. However, the distribution of this product for this purpose is prohibited because it is not authorized as a biocide.
Butyric acid is used in various industries. There is currently a lot of interest in using them as a precursor to biofuels, e.g. Can be used as biobutanol. Recently, research has focused on alternative fuel sources due to the rise in oil prices, along with the continued reduction in petroleum availability and the growing need for clean energy sources.
Butyric acid also finds numerous applications in the pharmaceutical and chemical industries. In the chemical industry, butyric acid is mainly used to produce cellulose acetate butyrate plastics.
Butyric acid has traditionally been used in analyzes as a key compound for ruminant milk fat, as it is not found in animal body fats or vegetable fats.
buy butyric acid?
Buy butyric acid at Laboratoriumdiscounter.nl. Not only because it is offered for a friendly price, but also because it is shipped quickly! Butyric acid available in different packaging at Laboratoriumdiscounter. Order butyric acid online with a few clicks and fast delivery!
Technical data
Butanoic acid
Empirical formula C4H8O2
Molar mass (M) 88,11 g/mol
Density (D) 0,96 g/cm³
Boiling point (bp) 165 °C
Flash point (flp) 71 °C
Melting point (mp) -5 °C
ADR 8 III
WGK 1
CAS No. 107-92-6
EG-Nr. 203-532-3
UN-Nr. 2820
Downloads
$$$$$
Hazard statements
H302 Harmful if swallowed
H314 Causes severe skin burns and eye damage
Precautionary statements - prevention
P270 Do not eat, drink or smoke when using this product
P280 Wear protective gloves/protective clothing/eye protection/face protection
Precautionary statements - response
P302+P352 IF ON SKIN: Wash with plenty of soap and water
P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing
P310 Immediately call a POISON CENTER/doctor