You have no items in your shopping cart
Citric acid, anhydrous ≥99.9 %, Ph . Eur. USP, FCC, Ultra Pure
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Citric Acid?
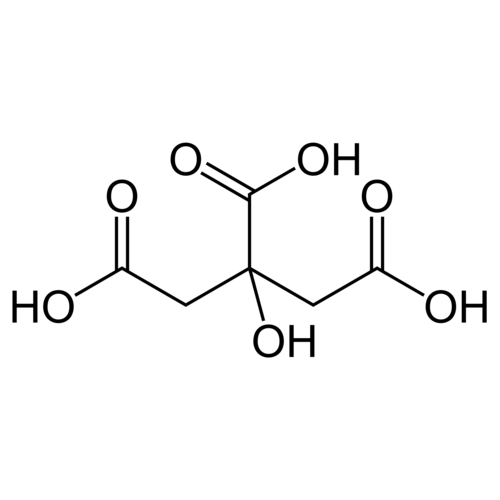
Citric acid (IUPAC name: 3-carboxy-3-hydroxypentanedioic acid) is an organic tricarboxylic acid present in most fruits, especially citrus fruits such as lemon, orange and tangerine. The molecular formula is C6H8O7.
It is a good preservative and natural antioxidant that is industrially added as a food additive in the processing and packaging of many foods, such as canned vegetable preserves.
In biochemistry, it appears as an intermediate metabolite in the tricarboxylic acid cycle, a process carried out by most living things.
What is citric acid used for?
-In Foods
Citric acid is used in powder form to prepare a lemon pepper seasoning.
Being one of the strongest edible acids, citric acid in food is mainly used as a preservative in foods and beverages and as a flavoring agent, especially in soft drinks and candies. In the European Union, citric acid when used as a food additive is designated by the number E330 (at FAO/WHO level it is identified by the number INS 330). Citrate salts of various metals are used to deliver those minerals in a bioavailable form in many dietary supplements. Citric acid has 247 kcal per 100 g. In the United States, the purity requirements for citric acid as a food additive are defined by the Food Chemicals Codex, published by the United States Pharmacopeia.
Citric acid can be added to ice cream as an emulsifier to prevent fats from separating, to caramel to prevent crystallization of sucrose, or in recipes in place of fresh lemon juice. Citric acid is used with sodium bicarbonate in a wide variety of effervescent formulations, both for ingestion (e.g., powders and tablets) and for personal care (e.g., bath salts, bath bombs, and grease cleansing). Citric acid sold in dry powder form is commonly sold in markets and supermarkets as "sour salt", due to its physical resemblance to table salt. It is used in culinary applications, as an alternative to vinegar or lemon juice where a pure acid is needed. Citric acid can be used in food coloring to balance the pH of a normally basic dye.
-Cleansing and chelating agent
Citric acid is an excellent chelating agent that binds metals making them soluble. It is used to remove scale build-up in boilers and evaporators. It can be used to treat water, making it useful for improving the effectiveness of laundry detergents and detergents. Chelating metals in hard water allows these cleaners to lather and work better without the need to soften the water. Citric acid is the active ingredient in some bathroom and kitchen cleaners. A 6 percent citric acid solution removes hard water stains from glass without scrubbing. Citric acid can be used in shampoo to remove wax and discoloration from hair.
-In cosmetics and hygiene products
Citric acid is used as an acidity regulator in creams, gels and liquids. Used in food and nutritional supplements, it can be classified as a processing aid if it is added for a technical or functional effect (e.g. acidity regulator, chelating agent, viscosity, etc.).
Citric acid is an alpha hydroxy acid and is an active ingredient in chemical skin peels.
Citric acid is used as one of the active ingredients in the production of facial tissues with antiviral properties.
-Other uses
The buffering properties of citrates are used to control pH in household cleaning products and pharmaceuticals.
Citric acid is used as an odorless alternative to white vinegar to dye fabrics with acid dyes.
Sodium citrate is a component of Benedict's reagent, used for both qualitative and quantitative identification of reducing sugars.
Citric acid can be used as an alternative to nitric acid in stainless steel passivation.
Citric acid can be used as a low odor stop bath as part of the photographic film developing process. Photographic developers are alkaline, so a mild acid is used to quickly neutralize and stop their action, but the commonly used acetic acid leaves a strong vinegar smell in the darkroom.
Citric acid/sodium potassium citrate can be used as a blood acid regulator.
Citric acid is an excellent soldering agent, both dry and in a concentrated solution in water. It must be removed after welding, especially on fine wires, as it is somewhat corrosive.
Buy citric acid?
If you need high quality anhydrous citric acid then you have come to the right place at Laboratiumdiscounter, not only is it both Foodgrade, Ph. Eur (pharmaceutical) but also Ultra Pure, which means that it is at least 99.9% pure and of the highest quality! Very pure citric acid for a friendly price! That is only possible at Laboratoriumdiscounter.nl!
How Can I Use Citric Acid Safely?
Citric acid is a mildly corrosive substance and it is therefore important that the necessary protective equipment is used. It can be irritating to the skin and especially in open wounds it can hurt a lot. It is therefore wise to wear at least gloves and glasses.
How can I store citric acid safely?
We recommend that you always store our products in the original packaging. It is a stable substance and will not decompose into other substances on its own. However, it can be corrosive to some metals, so plastic or glass is preferred.
Technical data
Molar mass (M) 192,13 g/mol
Density (D) 1,67 g/cm³
Melting point (mp) 153 °C
WGK 1
CAS No. 77-92-9
EG-Nr. 201-069-1
H319 Causes serious eye irritation
H335 May cause respiratory irritation
Precautionary statements
Precautionary statements - prevention
P261 Avoid breathing mist/vapours/spray
P280 Wear protective gloves/eye protection
Precautionary statements - response
P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact
lenses, if present and easy to do. Continue rinsing