You have no items in your shopping cart
Diethyl ether 99.9 +% Ph. Eur. Stabilized
- Buy 2 and save 5%
- Buy 6 and save 10%
This diethyl ether is of pharmaceutical quality (Ph. Eur) and stabilized with BHT.
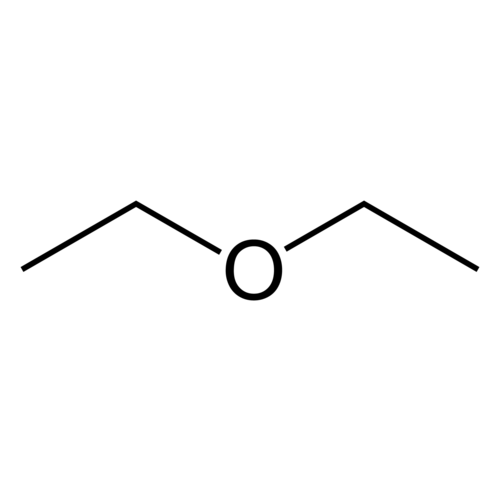
Diethyl ether, also called ethyl ether or ethoxyethane or simply ether (not to be confused with the family of ethers of which it is a part), is a clear, colorless and highly flammable liquid with a low boiling point and typical odor. Diethyl ether has the formula CH3-CH2-O-CH2-CH3. It is often used as a solvent. Diethyl ether has a high cetane number (85-96); and it is sparingly soluble in water.
-Use
-In medicine
Ether has been used as an anesthetic for a long time, but because of its very unpleasant side effects, such as nausea and its toxicity, as well as its high risk of dependence, it is no longer used in France and many developed countries. where less destructive anesthetics are preferred. It can also be used to remove the adhesive residue from an adhesive bandage on the skin.
-In chemistry
Ethyl ether is a solvent that is still used in the laboratory because it is a relatively inexpensive low polar organic solvent. It is often used as a solvent in various reactions, especially those with organometallic, where it has the role of stabilizer. Indeed, the doublets present on the oxygen stabilize the positive charge of the metal.
However, due to its extreme flammability and volatility, it is preferable to use a less hazardous solvent if possible.
It is also marketed as an aerosol as a heat engine starting aid.
-In biology
In biology, ethyl ether, commonly called "ether," is used to stun, put to sleep, or kill small insects you want to observe (such as Drosophila); we soak the felt with an etherizer and put the stopper on it before pouring the subjects into the funnel and we wait for the fumes to work.
Technical data:
Ether, Sulphuric ether, Ethyl ether
Empirical formula C4H10O
Molar mass (M) 74.12 g / mol
Density (D) 0.71 g / cm³
Boiling point (bp) 34.59 ° C
Flash point (flp) -40 ° C
Melting point (mp) -116 ° C
ADR 3 I.
WGK 1
CAS No. [60-29-7]
EC no. 200-467-2
UN No. 1155
$$$$$
Hazard statements
H224 Extremely flammable liquid and vapor
H302 Harmful if swallowed
H336 May cause drowsiness or dizziness
Safety recommendations
Precautions - prevention
P210 Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. Do not smoke.
P243 Take measures to avoid static discharge.
P261 Avoid breathing dust / fume / gas / mist / vapor / spray.
Precautions - response
P303 + P361 + P353 IF ON SKIN (or hair): Immediately contaminate clothing
pull out. Rinse skin with water [or shower].
P304 + P340 IF INHALED: Remove person to fresh air and keep comfortable for breathing.
P312 Call a POISON CENTER / doctor if you feel unwell.
Additional hazard information
EUH019 May form explosive peroxides.
EUH066 Repeated exposure may cause skin dryness or cracking.


%%%%%
| MSDS Diethylether (NL) |
| MSDS Diethyl ether (EN) |
| MSDS Éther diéthylique (FR) |
| MSDS Éter dietílico (ES) |