You have no items in your shopping cart
Ammonium acetate
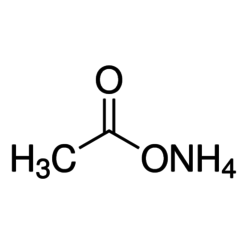
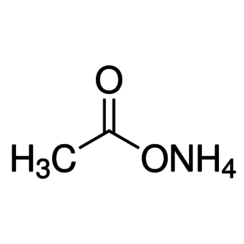
Ammonium acetate, also known as spirit of Mindererus in aqueous solution, is a chemical compound with the formula NH4CH3CO2. It is a white, hygroscopic solid and can be derived from the reaction of ammonia and acetic acid. It is available commercially
Uses
It is the main precursor to acetamide:
- NH4CH3CO2 → CH3C(O)NH2 + H2O
It is also used as a diuretic.
Buffer
As the salt of a weak acid and a weak base, ammonium acetate is often used with acetic acid to create a buffer solution. Ammonium acetate is volatile at low pressures. Because of this, it has been used to replace cell buffers with non-volatile salts in preparing samples for mass spectrometry. It is also popular as a buffer for mobile phases for HPLC with ELSD detection for this reason. Other volatile salts that have been used for this include ammonium formate.
Other
- a biodegradable de-icing agent.
- a catalyst in the Knoevenagel condensation and as a source of ammonia in the Borch reaction in organic synthesis.
- a protein precipitating reagent in dialysis to remove contaminants via diffusion.
- a reagent in agricultural chemistry for determination of soil CEC (cation exchange capacity ) and determination of available potassium in soil wherein the ammonium ion acts as a replacement cation for potassium.
Food additive
Ammonium acetate is also used as a food additive as an acidity regulator; INS number 264. It is approved for usage in Australia and New Zealand.