You have no items in your shopping cart
Formic acid 85 %
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Formic Acid?
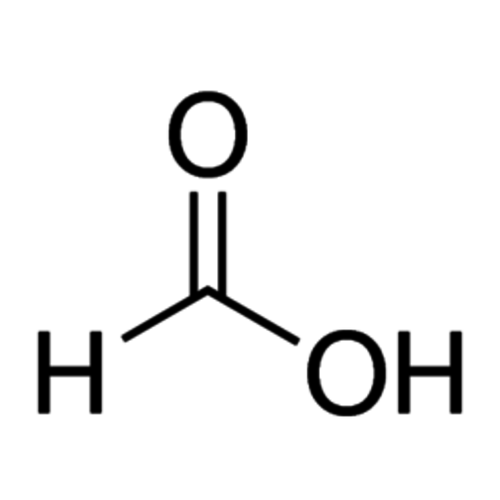
Formic acid (according to IUPAC methanoic acid nomenclature, Latin acidum formicum from formica 'ant') is a colorless, corrosive, and water-soluble liquid commonly used in nature by living things for defense purposes. With the semi-structural formula HCOOH, it is the simplest carboxylic acid and the shortest chain alkanoic acid; the carboxy group (-COOH) determines its properties particularly strongly. The carbon atom has an oxidation state of +2. It can therefore act as a hydride transfer analogous to the carbonyl compounds, hence the reducing effect. The salts of formic acid are called formates (systematically also methanoates) and have the semi-structural formula (HCOO) nM, where n corresponds to the valence of the metal ion. Examples of formates are sodium formate, HCOONa and aluminum formate, (HCOO) 3 Al. The esters of formic acid are also called formates.
What is Formic Acid used for?
Formic acid was used until 1998 under the E number E236 as a preservative in fish, fruit and vegetable products, but has since - unlike Switzerland - no longer been approved as a food additive in the EU. The related substances sodium and calcium formate are also no longer permitted as food additives (E237 and E238). In medicine it is used as an antirheumatic agent and for the treatment of vulgar warts. A ready-made solution containing formic acid is applied to the wart. In the textile and leather industry they are used for staining and impregnation. Sometimes it is also used as a disinfectant. It also kills good bacteria. In the chemical industry it is used to neutralize alkaline reaction mixtures. In electronics production, formic acid is used as a reducing agent in the soldering process. It is used industrially for descaling cooling water systems, as the resulting waste water contains only the harmless calcium formate with a low COD value.
In genetics, formic acid can be used in combination with the enzyme AP endonuclease to create random insertion mutants, known as in vitro mutagenesis. In the plastics industry it is used to bond polyamide plastics.
Concentrated formic acid is used to clean raw gems because it strongly attacks limestone and other impurities, exposing the gem without damaging it. This cleaning process should only be used with acid-resistant gemstones.
Scientists at the Leibniz Institute for Catalysis have experimentally achieved the catalytic release of hydrogen from formic acid even at room temperature. This hydrogen can be converted into electricity in fuel cells. This capability can be used for small-scale energy storage. The direct formation reaction of formic acid from hydrogen and carbon dioxide is thermodynamically very limited and the efficiency of the corresponding processes is therefore quite low.
buy formic acid?
You can buy pure formic acid at Laboratoriumdiscounter. Not only affordable but also delivered quickly. Available in different packaging and always with volume discount.
What Safety Precautions Should I Take Before Using Chemicals?
It is always wise to work as safely as possible when working with chemicals. Even substances that do not seem dangerous at first can cause enormous damage if they get into your eyes. Therefore, always wear safety goggles. You also want to prevent chemicals from ending up on your skin, which is why it is important to always use good gloves or disposable gloves.
Respiratory protection is necessary for volatile substances, vaporous liquids and solids that dust. There are many different types of filters, so you should always refer to the MSDS to find out which filter you need. Many filters also specify which substances they are for.
In some cases it may be necessary to protect the whole body, in which case a plastic overall is needed, you can find it here.
When working with flammable and oxidizing substances, it is important to always have fire extinguishers and absorbents at hand. It is also useful to be able to clean up spills of aggressive chemicals immediately with absorbents and to dispose of them in accordance with international and/or local legislation.
If you need more information on how to handle a specific substance, always consult the safety data sheet. You can find this on the product page or request it via [email protected]
Technical data:
Formylic acid, Methanoic acid, Hydrogen carboxylic acid
Empirical formula CH2O2
Molar mass (M) 46.02 g / mol
Density (D) 1.19 g / cm³
Boiling point (bp) 106 ° C
Flash point (flp) 65 ° C
Melting point (mp) 4 ° C
ADR 8 II
WGK 1
CAS No. [64-18-6]
EC no. 200-579-1
UN No. 1779
$$$$$
Hazard statements
H290 May be corrosive to metals
H302 Harmful if swallowed
H314 Causes severe burns and eye damage
H331 Toxic by inhalation
Precautions - prevention
P260 Do not breathe mist / vapor.
P280 Wear protective gloves / protective clothing / eye protection / face protection.
Precautions - response
P301 + P330 + P331 IF SWALLOWED: rinse mouth. DO NOT induce vomiting.
P303 + P361 + P353 IF ON SKIN (or hair): Immediately contaminate clothing
pull out. Rinse skin with water [or shower].
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for a period of time
amount of minutes; remove contact lenses, if possible; keep rinsing.
P310 Immediately call a POISON CENTER / doctor.
Supplemental Hazard Information
EUH071 Corrosive to the respiratory tract.


%%%%%
| MSDS Mierenzuur (NL) |
| MSDS Ameisensäure (DE) |
| MSDS Formic acid (EN) |
| MSDS Acide formique (FR) |
| MSDS Ácido fórmico (ES) |