You have no items in your shopping cart
Barium Nitrate 99.8 +% Extra pure
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Barium Nitrate?
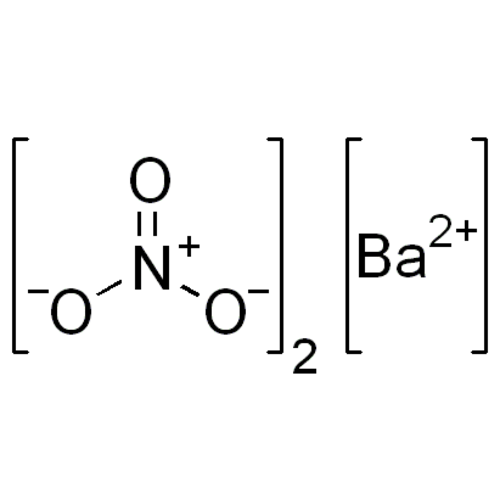
Barium nitrate is a nitrate salt of the alkaline earth metal barium. It has the formula Ba(NO3)2 and belongs to the group of nitrates.
Barium nitrate occurs as a colorless crystalline solid. It is harmful to health and a substance that is slightly hazardous to water. In a flame it produces the green flame color typical of barium and can be used as an oxidizing agent.
Barium nitrate decomposes at temperatures above 550 °C to form barium oxide, nitrogen, oxygen and nitric oxide. Due to the released oxygen and especially the released nitric oxide, barium nitrate is a good oxidizing agent.
Anhydrous barium nitrate crystallizes in the cubic crystal system in the space group Pa3 (space group No. 205) with the lattice parameter a = 811.84 pm. There are four formula units in the unit cell. The crystals are isotypical for strontium nitrate.
It is used in fluorescent screens for television receivers.
Buy Barium Nitrate?
You can buy barium nitrate at Laboratoriumdiscounter, high quality for a friendly price!
-Technical data
Empirical formula Ba(NO3)2
Molar mass (M) 261.35 g/mol
Density (D) 3.2
Melting point (mp)590 °C (Dec)
Solubility 90 g/l (H2O, 20 °C)
ADR 5.1 (6.1) II • WGK 1
CAS No.[10022-31-8]
EC-No. 233-020-5 • UN No. 1446
$$$$$
Hazard statements
H272 May intensify fire; oxidizing.
H301 Toxic if swallowed.
H319 Causes serious eye irritation.
H332 Harmful by inhalation.
Safety recommendations
Precautions - prevention
P221 Absolutely avoid mixing with combustible materials.
P261 Avoid breathing dust.
Precautions - response
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for a long period of time
amount of minutes; remove contact lenses, if possible; keep rinsing.
P312 Call a POISON CENTER / doctor if you feel unwell.
%%%%%