You have no items in your shopping cart
Potassium aluminum sulfate dodecahydrate 99+% extra pure
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Potassium Aluminum Sulfate?
Alum (from Middle High German alūn, from Latin alumen 'bitter clay salt', 'alum', from Indo-European alu- 'bitter'; English: Alum, French: Alun) was originally just a potassium aluminum salt (KAl(SO4) 2), the crystallized hydrous sulfuric acid double salt (combined metal sulfate) of potassium and aluminum (also called potassium aluminum sulfate). In the meantime, the corresponding ammonium aluminum salt is sometimes referred to, while the name alum applies to all sulfuric acid double compounds of the same chemical composition, where the metal used for potassium or aluminum is then put in front of the name, e.g. chromium alum for the sulfuric acid double salt of potassium and chromium.
Alums always have the composition MIMIII (SO4) 2 12 H2O, with MI monovalent metal cations such as the alkali metals sodium, potassium, rubidium and cesium - with the exception of lithium, as it cannot be absorbed without loss of stability - thallium or ammonium and its organic substituted derivatives; MIII can be the following triple positively charged metal cations: aluminum, gallium, indium, titanium, vanadium, chromium, manganese, iron, cobalt, rhodium, iridium, and sometimes thallium. The typical alum are those with aluminum, chromium and iron. All alum crystallizes in the cubic system, usually in the octahedral form and always with 12 molecules of crystal water. Furthermore, only two highly unstable sodium alums are known, aluminum and chrome alum.
What is Potassium Aluminum Sulphate used for?
In the tannery, alum is used to boil white the hides, in calico printing and in fabric dyeing they are used for mordant. It is also used for waterproofing materials that are then aspirated with oleic acid, for clarifying liquids, etc. In many cases, the alum must be completely free of iron, the presence of which is demonstrated by blood lye (blue color) . In papermaking, dyeing and white tanning, aluminum sulfate itself is now often used instead of alum, which is why it is often referred to as concentrated alum.
Ammonium alum (Alumen amoniacale), (NH4)Al(SO4)2 12 H2O, is produced like potassium alum by adding ammonium sulfate to aluminum sulfate instead of potassium sulfate. It contains 49.62% water of crystallization, is more soluble in cold water than ordinary potassium alum and is used as such. The content of anhydrous aluminum sulfate is 10.8% for potassium alum, 11.9% for ammonium alum and 15.4% for so-called concentrated alum (see above). Ammonia alum, which is often a mixture with potassium alum, gives off the pungent smell of ammonia (ammonia spirit) when treated with caustic potash.
Soda alum is rarely used because it weathers quickly, becomes cloudy and eventually crumbles to a white powder.
Chrome alum, KCr (SO4) 2 · 12 H2O (Alumen chromicum), in which the aluminum is replaced by chromium, is used in dyeing and tanning. It often arises as a waste product in the production of tar paint, in which chromic acid is used as an oxidizing agent, and consists of octahedra that are almost black when light is incident and dark red when light is transmitted, which dissolve in water with a violet color.
Alum is also used to make modeling clay. The most common application in everyday life is the alum bar, which is used as an astringent to stop bleeding. Alum has been used in medicine since ancient times and the Middle Ages.[5] But alum is also used in the garden. Here hydrangeas are fertilized with alum to give the flowers a purple or blue color. In Thailand, for example, it is added to the water to bind the suspended matter in it and to clarify the earth's water. It is also used as a deodorant (French: Pierre d'Alun). The alum is moistened and applied to the areas to be deodorized. Alum powder (Chinese 明矾粉, Pinyin míngfánfěn) has been used in China for centuries to make the fried breakfast pastries Youtiao (Chinese 油条, Pinyin yóutiáo).
Crystal growers often use potassium aluminum and potassium chromium alum. Both alums can be grown into centimeter-sized crystals.
buy alum?
You can buy the best quality Potassium Aluminum Sulphate at Laboratoriumdiscounter. Sharply spaced and delivered quickly. Always with volume discount and delivered throughout the EU.
Technical data
Alum, Potassium alum, Aluminum potassium sulphate
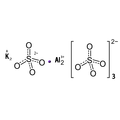
Empirical formula KAI(SO4)2 12 H2O
Molar mass (M) 474.39 g/mol
Density (D) 1.75 g/cm³
Melting point (mp) 92.5°C
WCK 1
CAS no. [7784-24-9]
EC-No. 233-141-3