You have no items in your shopping cart
p-Toluenesulphonic acid monohydrate ≥98 %, pure
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Toluene Sulphonic Acid?
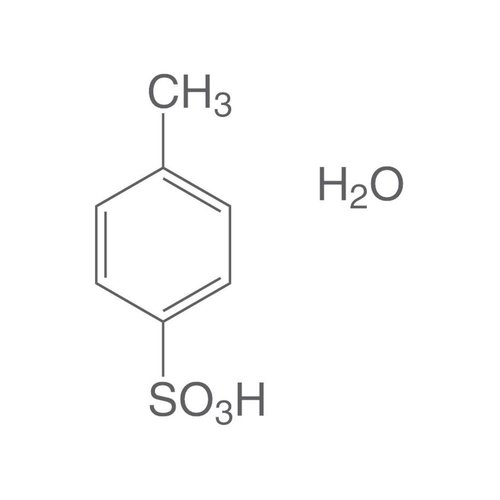
Paratoluene sulfonic acid (APTS) or tosylic acid is an organic compound with the molecular formula C7H8O3S. Consisting of a benzene ring substituted by a methyl group (toluene) and a sulfonic acid group at position 1,4, it is one of the three isomers of toluene sulfonic acid, the para compound. It is in the form of a white solid that is soluble in water, alcohols and other polar solvents. Using the abbreviated notation Ts or Tos to denote the tosyl group of formula CH3C6H4SO2-, the formula of the APTS is written in condensed form TsOH (or TosOH). It is generally found in monohydrate form with the formula TsOH H2O.
Like other sulfonic acids, APTS is a strong organic acid. By comparison, it is about a million times stronger than benzoic acid, another common organic acid. In addition, it is one of the rare strong acids that is present in solid form under "ordinary" experimental conditions, which can facilitate certain manipulations.
What is Toluene Sulphonic Acid used for?
In organic synthesis, APTS is typically used as an acid catalyst. Unlike sulfuric acid, it has no oxidizing character and can be advantageously substituted when solubility in the organic phase is desired. In addition, the conjugate base the tosylate anion (TsO-) is not nucleophilic, limiting side reactions.
The following reactions may benefit from acid catalysis by APTS:
esterification of carboxylic acids
ester transesterification
acetalization of aldehydes
Buy Toluene sulfonic acid?
If you need high-quality p-Toluene sulfonic acid, you have come to the right place at Laboratoriumdiscounter. Delivered quickly and for a friendly price. Available in different packaging.
Technical information
Paratoluolsulphonic acid
Empirical formula C7H8O3S · H2O
Molar mass (M) 190,22 g/mol
Boiling point (bp) 140 °C
Flash point (flp) >150 °C
Melting point (mp) 56 °C
ADR 8 III
WGK 1
CAS No. [6192-52-5]
EG-Nr. 203-180-0
UN-Nr. 2585
Downloads
$$$$$
Hazard statements
H315 Causes skin irritation
H319 Causes serious eye irritation
H335 May cause respiratory irritation
Precautions - response
P302 + P352 IF ON SKIN: Wash with plenty of water.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for a long period of time
amount of minutes; remove contact lenses, if possible; keep rinsing.