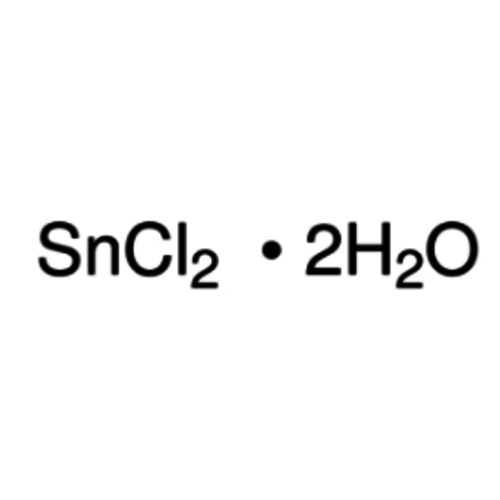You have no items in your shopping cart
Tin(II) chloride dihydrate 99+% extra pure
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Stannous Chloride?
Tin(II) chloride is a white crystalline solid whose formula is SnCl2. It forms a stable dihydrate, but aqueous solutions tend to undergo hydrolysis, especially when heated. SnCl2 is widely used as a reducing agent (in acid solution) and in electrolytic baths for tin coating. Tin(II) chloride should not be confused with other tin chlorides such as Tin(IV) chloride (SnCl4).
What is Tin(II) chloride used for?
Tin(II) chloride is used as a reducing agent in the laboratory. In electroplating, it is used for electrolytic tin plating.
Tin(II) chloride is used in chemical analysis, in dyeing to reduce indigo and iron and manganese oxide on witnesses, as a mordant, namely for cochineal dyeing, for brightening and brightening, also for displaying golden purple and lacquer colors , as anti-chlorine and for removing rust stains from laundry.
Tin(II) chloride, dissolved in concentrated hydrochloric acid, is used as a detecting agent for arsenic in the Bettendorf sample. The solution turns brown because stannous chloride reduces the arsenic compound and precipitates elemental arsenic. The only interfering elements are mercury and precious metals. This reagent can also be used to detect sesame oil because the solution turns red when layered with sesame oil.
Buy tin(II) chloride dihydrate?
If you need stannous chloride, you have come to the right place at Laboratoriumdiscounter. Our Tin(II) chloride is very pure and that for a friendly price! Available in different packaging, always with volume discount.
Technical data
Empirical formula SnCl2 · 2 H2O
Molar mass (M) 225,63 g/mol
Density (D) 2,71
Boiling point (bp) 623 °C
Melting point (mp)38 °C
Solubility 1780 g/l (H2O, 10 °C)
ADR 8 II • WGK 1
CAS No.[10025-69-1]
EG-Nr. 231-868-0 • UN-Nr. 3260
Downloads
$$$$$
Hazard Statements
H302 Harmful if swallowed.
H314 Causes severe skin burns and eye damage.
H317 May cause an allergic skin reaction.
H412 Harmful to aquatic life with long lasting effects.
Precautions - Prevention
P273 Avoid release to the environment.
P280 Wear protective gloves/protective clothing/eye protection/face protection.
Precautions - Response
P302+P352 IF ON SKIN: Wash with plenty of water.
P305+P351+P338 IF IN EYES: Rinse cautiously with water for
amount of minutes; remove contact lenses, if possible; keep rinsing.
P308 NA (Possible) Exposure:.
P310 Immediately call a POISON CENTER/doctor.
Precautions - Storage
P405 Keep locked up.