You have no items in your shopping cart
Luminol 99+%, pure
- Buy 2 and save 5%
- Buy 6 and save 10%
Luminol is a chemical that produces beautiful blue fluorescence upon oxidation by hydrogen peroxide. In addition to one of the best known examples of chemiluminescence, it is also a valuable tool for investigating crime scenes whose blue glow reveals the presence of blood.
For teachers, demonstrating the luminol response can contribute to discussions about oxidation reduction reactions, energy savings and electron energy levels. The following demonstration is ideal for high and high school students.
Materials
1 g of Luminol
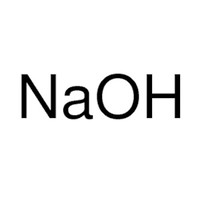
20 ml sodium hydroxide solution (1 M)
10 ml hydrogen peroxide (3%)
0.2 g of potassium ferricyanide
piece of rubber hose
Funnel
Ring stand
Tripod
Clamps
Preparation
You need a separate cup for each of the 2 stock solutions you are going to prepare. Prepare the solutions immediately before use. Put on a lab coat or apron, glasses and gloves before preparing solutions. Note: hydrogen peroxide is a strong oxidizer. Avoid skin contact. Sodium hydroxide and its solutions are corrosive and may irritate the skin. Avoid skin contact.
To prepare stock solution A, fill a beaker with 100 ml of water. Add 0.18 g of luminol and 3.0 ml of sodium hydroxide solution (1 M).
To prepare stock solution B, fill another beaker with 100 ml of water. Add 1 ml of hydrogen peroxide (3%) and 0.03 g of potassium ferricyanide.
Experiment
DIM the lights.
At the same time, pour an equal amount of solution A and solution B into the funnel.
While the 2 solutions are mixing, a blue light is emitted that is relatively bright and should last several minutes.
Technical information
Fluorescence indicator, peroxidase reagent
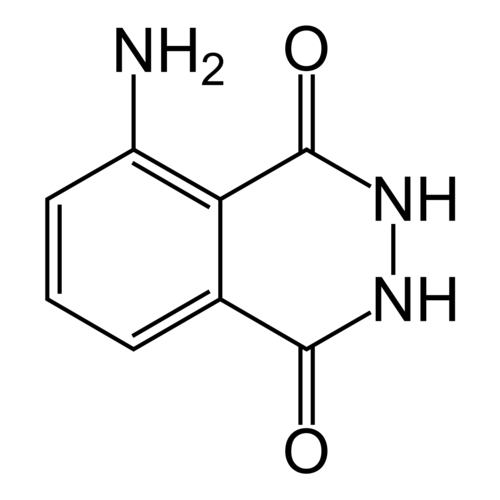
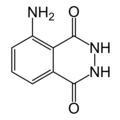
3-Aminophthalic hydrazide, 5-Amino-2,3-dihydro-phthalazine-1,4-dione
Empirical formula C8H7N3O2
Molar mass (M) 177.16 g / mol
Melting point (mp) approx. 320 ° C
WGK 1
CAS No. [521-31-3]
EC no. 208-309-4
Downloads
$$$$$
Hazard statements
H302 Harmful if swallowed.
H315 Causes skin irritation.
H319 Causes serious eye irritation.
H335 May cause respiratory irritation.
Precautions - prevention
P280 Wear protective gloves / eye protection.
Precautions - response
P302 + P352 IF ON SKIN: Wash with plenty of soap and water.
P304 + P340 IF INHALED: Remove to fresh air and keep at rest in a position suitable
makes breathing easier.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for a period of time
amount of minutes; remove contact lenses, if possible; keep rinsing.