You have no items in your shopping cart
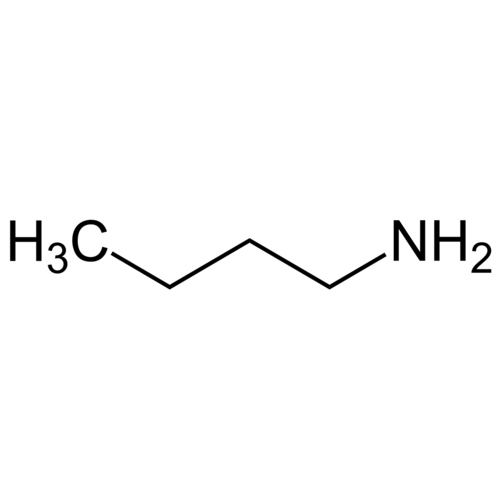
n-butylamine 99.9 +% extra pure
- Buy 2 and save 5%
- Buy 10 and save 10%
What is Butylamine?
n-Butylamine is a chemical compound produced in the chemical and pharmaceutical industries as an intermediate in the synthesis of organic substances. It is a primary amine. n-Butylamine belongs to the group of aliphatic amines, more specifically the butylamines, and is isomeric for isobutylamine, sec-butylamine and tert-butylamine.
The common name Norvalamin is derived from the amino acid Norvaline. Decarboxylation of norvaline produces n-butylamine.
n-Butylamine is formed when n-butanol or n-butanal is reacted with ammonia in the presence of catalysts. It can also be generated from valeric acid by the so-called Schmidt reaction.
n-Butylamine forms highly flammable vapour-air mixtures. The compound has a flash point at −14 °C. The explosion range is between 1.7 volume percent (50 g m - 3) as lower explosive limit (LEL) and 10 volume percent (304 g m - 3) as upper explosive limit (UEL) . The maximum gap width was determined to be 0.92 mm. This results in an assignment to explosion group IIA. The ignition temperature is 310 ° C. The substance therefore falls into temperature class T2.
What is Butylamine used for?
n-Butylamine is an intermediate in the production of various organic compounds such as dyes, drugs, pesticides, plasticizers, emulsifiers, anti-corrosion agents and adhesives and can be used as a solvent in titration.
Buy Butylamine?
Butylamine of the best quality can be found at Laboratoriumdiscounter.nl Not only a fair price but also fast shipping. N-butylamine is available at Laboratoriumdiscounter in different packaging. Always with volume discount!
Technical information
Empirical formula C4H11N
Molar mass (M) 73,14 g/mol
Density (D) 0,74 g/cm³
Boiling point (bp) 77 °C
Melting point (mp) -49 °C
WGK 1
CAS No. [109-73-9]
ADR 3 (8) II UN1125
Downloads
$$$$$
Hazard statements
H225 Highly flammable liquid and vapor.
H302 Harmful if swallowed.
H311 + H331 Toxic in contact with skin or if inhaled. H314 Causes severe skin burns and eye damage.
Precautionary statements
Prevention
P210 Keep away from heat / sparks / open flames / hot surfaces. No smoking.
P233 Keep container tightly closed.
P240 Ground / bond container and receiving equipment.
P280 Wear protective gloves / protective clothing / eye protection / face protection. Response
P301 + P330 + P331 IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
P302 + P352 IF ON SKIN: Wash with plenty of soap and water.
P304 + P340 IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuous rinsing.
P308 + P310 IF exposed or concerned: immediately call a POISON CENTER or doctor / physician.
Storage
P403 + P235 Store in a well-ventilated place. Keep cool.