You have no items in your shopping cart
Potassium Permanganate 98.5+% Pure
- Buy 2 and save 5%
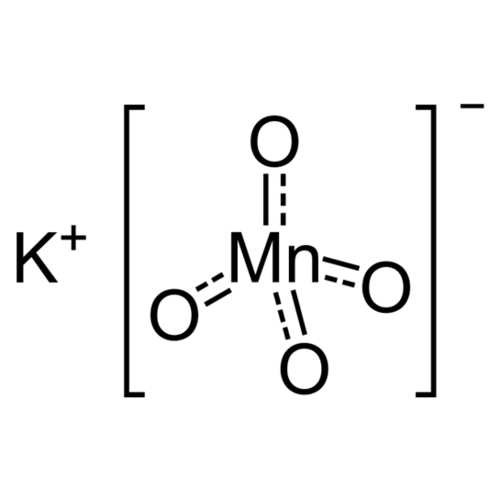
Potassium permanganate is an anhydrous, solid, ionic chemical compound of potassium K+ ions and MnO4 permanganate ions with the chemical formula KMnO4. The manganese atoms are in oxidation state +VII, so the name according to IUPAC is potassium manganate (VII). Being highly soluble in water, it is an important salt in inorganic chemistry, especially in manganimetry or analytical determination by the permanganate ion.
Potassium permanganate KMnO4 is a powerful oxidizing agent. In the solid state, it appears as purple crystals with a metallic sheen.
It decomposes thermally at 240°C into manganese dioxide and oxygen gas.
The aqueous solution, which is highly soluble in water, is purple in color and increases in intensity with concentration.
Solid potassium permanganate is a very strong oxidizing agent. A very exothermic reaction occurs when mixed with glycerin. This can result in spontaneous combustion which can melt glass and a large number of containers and ignite any combustible materials in the vicinity. This type of reaction can occur when solid potassium permanganate KMnO4 is contacted with a large number of organic compounds. Aqueous solutions of KMnO4, and especially dilute solutions, are much less dangerous. Potassium permanganate should never be stored near petroleum or any easily oxidizable substance.
Potassium permanganate can stain clothing or skin, it reacts with skin or fabric, similar to reducing bodies for this oxidizing body, and leaves traces of manganese dioxide, causing brown or brown spots on the skin. More seriously, it gnaws deeply at the contaminated mucous membranes and walls of the digestive tract to the stomach if accidentally ingested. This substance and its fresh aqueous solutions should be handled with care. Stains on clothing can be cleaned with iron(II) sulphate or oxalic acid. The spots on the skin disappear after about 3 weeks or even 1 month and in some cases even more. Nail stains can be removed with oxalic acid.
Oxidation and Synthesis - Organic Chemicals and Intermediates
manufacture. Oxidizes impurities in organic and inorganic
Chemicals.
Water Treatment - Oxidizes Iron, Manganese and
hydrogen sulfide; regulates taste and smell; and is an alternative
pre-oxidant for disinfection by-product (THMs and HAAs)
check.
Municipal Wastewater Treatment - Destroys Hydrogen
sulphide in waste water and sludge. Improves sludge dewatering.
Industrial Wastewater Treatment - Oxidizes Hydrogen
sulfide, phenols, iron, manganese and many other organic and
inorganic contaminants; resulting manganese dioxide aids in heavy metal removal.
Surface treatment of metal - Mill scale and dirt condition to
facilitate subsequent removal by acid pickling in wrought metals
production and jet engine cleaning.
Equipment Cleaning - Helps to clean organic and inorganic
residues from refining and cooling towers and other processing
equipment. Decontaminate hydrogen sulfides, pyrophoric iron
sulfides, phenols and others.
Purification of gases - Removes traces of impurities of sulfur,
arsine, phosphine, silane, borane and sulfides from carbon dioxide
and other industrial gases.
Mining and Metallurgical - Aid in Molybdenum Separation
of copper; removes impurities from zinc and cadmium; oxidizes
flotation connections. Removes iron and manganese from the acid mine
drainage.
Slug Quenching - Controls Hydrogen Sulfide and Acetylene
emissions during the quenching of hot slag.
Food Processing - Controls sulfides, soluble animal oil, fat,
organic acids, ketones, nitrogen compounds, mercaptans and
BID.
Technical data:
Empirical formula KMnO4
Molar mass (M) 158.04 g/mol
Density (D) 2.7 g/cm
Melting point (mp) >240 °C
ADR 5.1 II
WGK 3
CAS no. [7722-64-7]
EC-No. 231-760-3
UN No. 1490
Downloads
$$$$$
Hazard statements
H272 May intensify fire; oxidizer
H302 Harmful if swallowed
H314 Causes severe skin burns and eye damage
H361d Suspected of damaging the unborn child
H373 May cause damage to organs (brain) through prolonged or repeated exposure
(if inhaled)
H410 Very toxic to aquatic life with long lasting effects
Precautionary statements
Precautionary statements - prevention
P220 Keep away from clothing and other combustible materials
P273 Avoid release to the environment
P280 Wear protective gloves/eye protection