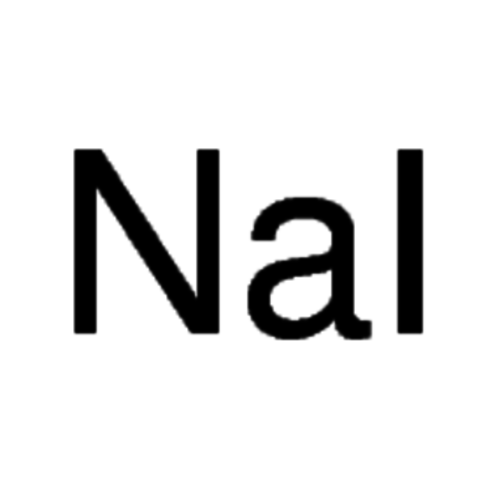You have no items in your shopping cart
Sodium iodide 99+% USP, Ph. Eur
- Buy 2 and save 5%
What is Sodium Iodide?
Sodium iodide is a white, crystalline salt with the molecular formula NaI that is used to detect ionizing radiation, treat iodine deficiency, and produce iodoalkanes in the Finkelstein reaction.
What is Sodium Iodide used for?
Sodium iodide is commonly used to treat and prevent iodine deficiency. The radioactive isotopes 123 iodine and 131 iodine are used as sodium iodide for nuclear medicine diagnostics, especially for thyroid scintigraphy. 131Iodine is used as sodium iodide as part of therapy with radioactive iodine as a therapeutic agent.
Another field of application is the Finkelstein reaction. Here an alkyl chloride or bromide is treated with sodium iodide in acetone.
The better leaving group iodide displaces, the worse leaving group chloride. The driving force of the reaction is the low solubility of sodium chloride in acetone, shifting the equilibrium to the alkyl iodide side. In addition to acetone, THF and acetonitrile can also be used as solvents.
Furthermore, there are numerous reactions analogous to the Finkelstein reaction, e.g. the preparation of trimethylsilyl iodide from trimethylsilyl chloride, acetyl iodide from acetyl chloride and much more.
In crystals doped with thallium (NaI:Tl+) on part of the sodium positions (NaI:Tl+), photons are generated by ionizing radiation and can thus be used as a scintillation detector, traditionally in nuclear medicine, geophysics, nuclear physics, etc. NaI:Tl+ is the most commonly used scintillation material because it produces the most light. The crystals are usually coupled to a photomultiplier and hermetically sealed since NaI is hygroscopic. Some parameters (beam hardness, afterglow, transparency) can be influenced by controlling the conditions under which the crystal grows. Higher doping crystals are used as X-ray detectors with high spectroscopic quality. Sodium iodide can be used as a single crystal or polycrystalline.
buy sodium iodide?
Are you looking for the best quality Sodium Iodide? Then you have come to the right place at Laboratorydiscounter. Our sodium iodide is not only of very pure quality, but also food grade and pharmaceutical certified. So at Laboratoriumdiscounter you are assured of the best quality sodium iodide. Delivered quickly and always in stock. Available in different packages and you always receive a volume discount when purchasing multiple packages.
Technical data
Empirical formula NaI
Molar mass (M) 149,89 g/mol
Density (D) 3,5 g/cm³
Boiling point (bp) 1304 °C
Melting point (mp) 651 °C
ADR 9 III
WGK 3
CAS No. [7681-82-5]
EG-Nr. 231-679-3
UN-Nr. 3077
Downloads
$$$$$
Hazard statements
H315 Causes skin irritation
H319 Causes serious eye irritation
H372 Causes damage to organs (thyroid gland) through prolonged or repeated exposure (if swallowed)
H400 Very toxic to aquatic life
Precautionary statements
Precautionary statements - prevention
P273 Avoid release to the environment
P280 Wear protective gloves/eye protection
Precautionary statements - response
P391 Collect spillage