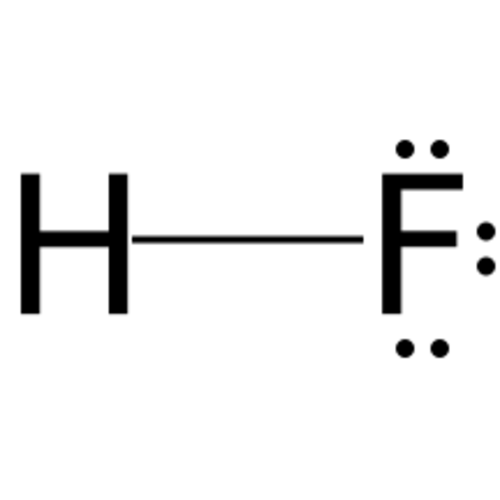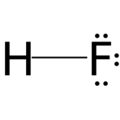You have no items in your shopping cart
Hydrogen fluoride 40%
- Buy 2 and save 5%
- Buy 6 and save 10%
Please note, we do not sell this product to private individuals. We consider the product to be so dangerous that it can only be used professionally.
Almost any inorganic oxide can be dissolved in concentrated solutions of hydrogen fluoride. The solubility of the oxides can be attributed to the fact that both the anion (in the form of water) and the cation (in the form of a polyfluoro complex) of the oxide do not exist freely in the solution. Hydrogen fluoride is therefore often used:
as an etchant in the glass industry (it reacts with glass - it is the only agent by which borosilicate glass can be broken down - and thus cannot be stored in it)
in the semiconductor industry
to purify aluminum and uranium
as a starting product for fluoride-containing substances (Teflon, CFCs)
as a solvent of oxides on the surface of aircraft turbine parts (mainly nickel-chromium, NiCr) to improve the quality of subsequent soldering processes or to strip coatings
as a solvent in laboratories to keep various substances in solution
In dilute solutions of hydrogen fluoride, it is often used as a rim cleaner.
Liquid anhydrous hydrogen fluoride is used as a catalyst in oil refineries during the production of high octane gasoline from the C4 fraction.
In organic synthesis, it is sometimes used in making organic fluorine compounds. Inorganic fluorine salts can also be prepared with it.
Technical product information:
Molar mass (M) 20,01 g/mol
Density (D) 1,18 g/cm³
Boiling point (bp) 108 °C
Melting point (mp) -35 °C
ADR 8 II
WGK 2
CAS No. 7664-39-3
EG-Nr. 231-634-8
UN-Nr. 1790
Downloads
$$$$$
Health and Safety
Hazard statements
H290 - May be corrosive to metals
H314 - Causes severe burns and eye damage
H300 + H310 + H330 - Fatal if swallowed, in contact with skin or if inhaled
Safety recommendations
P260 - Do not breathe dust / fume / gas / mist / vapor / spray
P262 - Avoid contact with eyes, skin, or clothing
P280 - Wear protective gloves / protective clothing / eye protection / face protection
P304 + P340 - IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P305 + P351 + P338 - IF IN EYES: Rinse cautiously with water for several minutes. remove contact lenses, if possible; keep rinsing
P310 - Immediately call a POISON CENTER or doctor
P303 + P361 + P353 - IF ON SKIN (or hair): Take off immediately all contaminated clothing. Skin with
rinse off water or shower