You have no items in your shopping cart
Magnesium sulphate
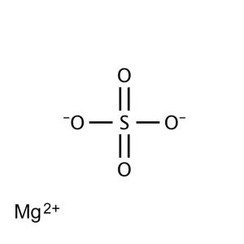
Magnesium sulfate is a chemical compound, a salt with the formula MgSO
4, consisting of magnesium cations Mg2+
(20.19% by mass) and sulfate anions SO2−
4. It is a white crystalline solid, soluble in water but not in ethanol.
Magnesium sulfate is usually encountered in the form of a hydrate MgSO
4•nH
2O, for various values of n between 1 and 11. The most common is the heptahydrate MgSO
4·7H
2O, known as Epsom salt, which is a household chemical with many traditional uses, including bath salts.[1]
The main use of magnesium sulfate is in agriculture, to correct soils deficient in magnesium (an essential plant nutrient). The monohydrate is favored for this use; by the mid-1970s, its production was 2.3 million tons per year. The anhydrous form and several hydrates occur in nature as minerals, and the salt is a significant component of the water from some springs.